SUMMARY
While integrin αvβ3 expression is linked to breast cancer progression its role in epithelial development is unclear. Here, we show that αvβ3 plays a critical role in adult mammary stem cells (MaSCs) during pregnancy. Whereas αvβ3 is a luminal progenitor marker in the virgin gland, we noted increased αvβ3 expression in MaSCs at mid-pregnancy. Accordingly, mice lacking αvβ3 or expressing a signaling deficient receptor showed defective mammary gland morphogenesis during pregnancy. This was associated with decreased MaSC expansion, clonogenicity and expression of Slug, a master regulator of MaSCs. Surprisingly, αvβ3 deficient mice displayed normal development of the virgin gland with no effect on luminal progenitors. TGFβ2 induced αvβ3 expression leading to Slug nuclear accumulation and MaSC clonogenicity. In human breast cancer cells αvβ3 was necessary and sufficient for Slug activation, tumorsphere formation and tumor initiation. Thus, pregnancy-associated MaSCs require a TGFβ2/αvβ3/Slug pathway, which may contribute to breast cancer progression and stemness.
INTRODUCTION
In the adult mammary gland an epithelial hierarchy has been characterized that involves the step-wise differentiation of mammary stem cells (MaSC)/progenitor cells toward mature luminal and basal/myoepithelial cell fates (Visvader, 2009). MaSC/progenitor cells share a number of biological and biochemical properties with highly invasive breast cancer cells (Visvader, 2009) and thus may act as the cells-of-origin for more aggressive types of breast cancer (Jeselsohn et al., 2010; Lim et al., 2009). Molecular profiling of mammary cells at distinct stages of differentiation identified gene signatures associated with particular MaSC and progenitor cells (Lim et al., 2010; Pece et al., 2010). The MaSC gene expression signature, in particular, correlates with tumors that are less differentiated (Lim et al., 2010) and represent clinically advanced disease (Pece et al., 2010). In some breast cancers, a sub-set of tumor cells has been identified that share similar gene expression (Pece et al., 2010) and behavioral properties (Al-Hajj et al., 2003) with normal MaSCs, and are referred to as cancer stem cells (CSCs).
Integrins act as key cell surface receptors regulating adhesion-dependent functions critical for MaSC/progenitor behavior (Taddei et al., 2008) and breast carcinogenesis (Desgrosellier and Cheresh, 2010). Integrin αvβ3, in particular, is expressed in some of the most highly malignant tumor cells in carcinomas of the breast, pancreas, lung and prostate (Desgrosellier and Cheresh, 2010) where it may play an anchorage-independent role in tumor progression (Desgrosellier et al., 2009). Expression of β3 (CD61) in breast carcinoma cells promotes both lymph node (Desgrosellier et al., 2009) and bone metastases (Felding-Habermann et al., 2001; Liapis et al., 1996; Sloan et al., 2006; Takayama et al., 2005) and serves as a marker of CSCs in some murine breast cancer models (Vaillant et al., 2008). In the normal murine mammary gland surface β3 represents a marker of luminal progenitor cells (Asselin-Labat et al., 2007) and may be expressed on MaSCs (Bai and Rohrschneider, 2010), particularly in response to steroid hormones (Joshi et al., 2010). This suggests that αvβ3’s function in carcinoma cells may be related to a role in normal MaSC/progenitor cell behavior.
The epithelial hierarchy in the adult mammary gland represents a well-characterized system with rigorously defined markers (Asselin-Labat et al., 2007; Shackleton et al., 2006; Stingl et al., 2006) allowing us to characterize a possible role for αvβ3 in this process. Here we describe a role for αvβ3 in regulating Slug activation in MaSCs leading to MaSC expansion and mammary gland remodeling during pregnancy. Interestingly, αvβ3 also promotes Slug activation, anchorage-independent growth and tumor initiation in human breast cancer cells, hallmarks of tumor stemness.
RESULTS
β3 is required for mammary gland development during pregnancy
Previous studies showed β3 surface expression in luminal progenitors and some MaSCs from dissociated virgin mammary glands (Asselin-Labat et al., 2007). Consistent with these findings, we observed β3 expression in basal cells and a subset of luminal cells within the ducts of adult virgin mice (Figure 1A) that was confirmed by co-staining with basal and luminal markers (Figure S1A). These results show that the β3 expression pattern in the intact adult mammary gland is consistent with a potential role for β3 in luminal progenitors and MaSCs.
Figure 1. β3 is specifically required for mammary gland development during pregnancy.
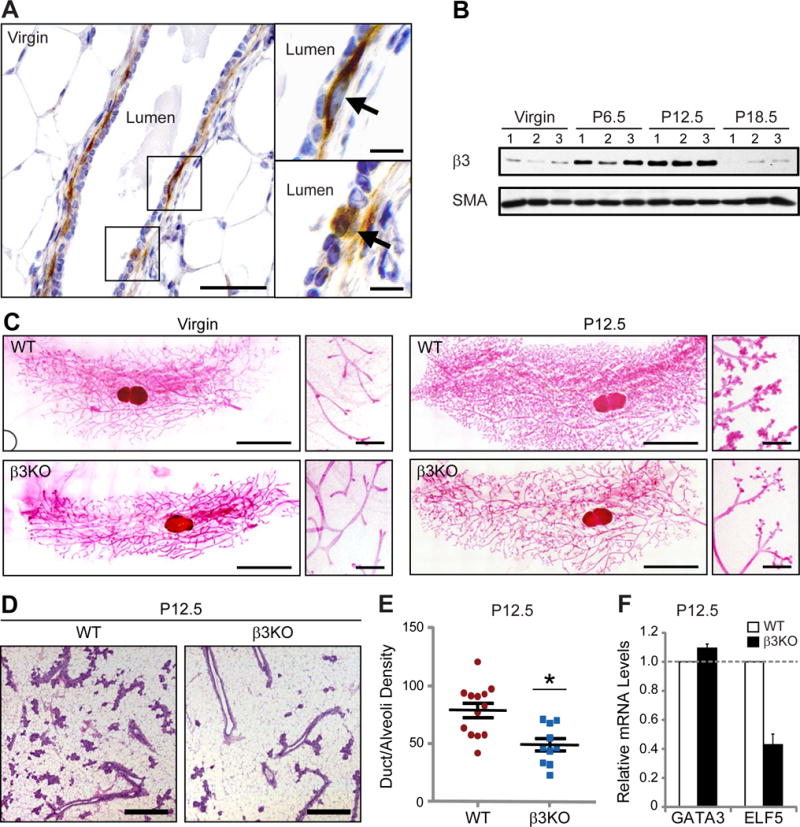
(A) Representative images of β3 immunohistochemistry in an adult virgin murine mammary gland. Shown is an example of a duct (left panel) with areas in boxes shown at high-power (right panels). Images on right show β3-expressing cells (arrows) in the basal epithelial cell layer (top, right) and a subset of luminal epithelial cells (bottom, right). Scale bars, 50 μm (left panel) and 10 μm (right panels).
(B) Western blot of whole mammary gland lysates for β3 and α-smooth muscle actin (SMA) (loading control). n=3 mice for each stage.
(C) Mammary gland whole-mounts from virgin and P12.5 WT and β3KO mice. Virgin; WT, n=8, β3KO, n=7, P12.5; WT, n=19, β3KO, n=10. Scale bars, 5 mm (low magnification), and 500 μm (high magnification).
(D) Representative H&E-stained sections from WT and β3KO P12.5 mammary glands. Scale bars, 500 μm.
(E) Quantitation of duct/alveoli density in P12.5 WT versus β3KO H&E-stained mammary gland sections. WT, n=13, β3KO, n=10, P=0.015. Data shown represent the mean ± s.e.m. and were analyzed by Student’s T-test. *P<0.05.
(F) qPCR results displaying the relative amount of GATA3 and ELF5 mRNA in WT and β3KO P12.5 mammary glands. WT, n=11, β3KO, n=9. Each sample was run in triplicate and GAPDH was used as a loading control. Data is displayed as the mean ± s.d. fold change (2−ΔΔCT) in β3KO glands relative to WT. See also Figures S1 and S2.
The adult murine mammary gland is a highly dynamic organ, constantly changing in response to hormones released during the estrus cycle and pregnancy. Analysis of β3 in whole-mammary gland lysates showed no differences during the estrus cycle (Figure S1B); however, relative to virgin glands, we observed increased β3 expression during early and mid-pregnancy that declined by late pregnancy (Figure 1B). Notably, the peak levels of β3 at pregnancy day 12.5 (P12.5) coincide with the maximum number of MaSCs reported during pregnancy (Asselin-Labat et al., 2010).
β3 expression in glands from both virgin and pregnant mice suggests a potential function for this receptor in mammary gland morphogenesis at either stage. To address this possibility, we examined the morphology of mammary gland whole-mounts from virgin and P12.5 wild-type (WT) and β3 knock-out (β3KO) mice, which lack β3 expression in the mammary gland (Figure S1C). While no differences were observed in mammary glands from virgin β3KO mice (Figure 1C; left panels), marked differences were seen in β3KO mammary glands at P12.5 which demonstrated fewer fine branches and alveolar buds relative to WT glands (Figure 1C; right panels). Importantly, this defect was maintained throughout late-stage pregnancy and lactation (Figure S1D,E) and correlated with decreased viability of litters born to β3KO dams (Figure S1F). However, we noted no difference in the ability of β3KO alveoli to produce milk at any stage examined (Figure S2A). Quantification of duct/alveoli density in H&E-stained sections showed a nearly 40% decrease in the density of P12.5 β3KO glands relative to WT controls (Figure 1D,E), consistent with the qualitative assessment of these glands. Thus, β3 appears to be required for mammary development during pregnancy but not for ductal morphogenesis in the virgin adult gland.
To discern a potential mechanism that accounts for this phenotype, we assessed the relative amounts of epithelial cell proliferation, apoptosis and differentiation in WT and β3KO P12.5 mammary glands. Quantitative RT-PCR from whole mammary glands showed reduced mRNA levels of the alveolar marker ELF5 (57% decrease), but not the luminal differentiation marker GATA3 in P12.5 β3KO mammary glands relative to WT controls (Figure 1F). In contrast, we were unable to detect any effect on proliferation (Figure S2B,C) or apoptosis, which was essentially absent from the P12.5 gland (data not shown). Importantly, we noted similar levels of nuclear ELF5 protein in β3KO alveoli compared to those from WT mice (Figure S2D), indicating that the decreased ELF5 mRNA levels observed in P12.5 β3KO mammary glands is consistent with fewer alveoli and not due to dysregulated ELF5 expression. Taken together, these data show that β3 deletion is associated with defective initiation of alveologenesis during pregnancy, suggesting that β3KO mice may display a defect in MaSC/progenitor cells.
Pregnancy is associated with increased β3 expression in MaSCs
Mammary gland remodeling and differentiation during pregnancy requires the coordinated response of multiple cell types including MaSCs and progenitors (Asselin-Labat et al., 2010; Jeselsohn et al., 2010; van Amerongen et al., 2012; Van Keymeulen et al., 2011). To determine which MaSC/progenitor cell types might require β3 during pregnancy we first compared β3 expression in WT virgin and P12.5 mammary glands by flow cytometry. Analysis of live (propidium iodide negative) lineage negative (Lin−; CD31−, CD45−, Ter19−) mammary cells for surface expression of CD24 and CD29 (β1 integrin) identify enriched populations of mature luminal and progenitor cells (CD24+CD29lo) as well as basal and MaSCs (CD24+CD29hi) in both virgin (Shackleton et al., 2006) and P12.5 mammary glands (Asselin-Labat et al., 2010). We then evaluated surface β3 levels in these live Lin−CD24+CD29hi/lo cells to determine the MaSC/progenitor cells that express β3 during pregnancy. We found that most β3+ cells in the virgin gland resided in the CD29lo population, and the percentage of β3+CD29lo cells decreased during pregnancy, as previously described (Asselin-Labat et al., 2007) (Figure 2A,B) though some β3+ luminal cells remain (Figure S3A,B). However, to our surprise, we observed increased β3 surface levels on the P12.5 CD29hi MaSC-enriched population compared to the virgin gland (Figure 2A,B and S3A,B). Interestingly, this effect is concurrent with the expansion of the CD29hi MaSC pool at this stage (Figure 2A) (Asselin-Labat et al., 2010). This increased β3 level was transient, as it decreased to that of virgin glands after full involution (8 weeks) (Figure S3C). The increased β3 surface levels in P12.5 CD29hi cells corresponded to a nearly 4-fold induction of β3 mRNA compared to virgin CD29hi cells, with little effect on αv levels (Figure 2C), suggesting that this effect is due to enhanced β3 expression in these cells. Accordingly, P12.5 β3+CD29hi cells expressed basal markers (Figure 2D and Figure S3D) consistent with β3 co-staining with basal markers in P12.5 mammary sections (Figure S3B). These data show that β3 expression is dynamically regulated in CD29hi MaSC/basal cells at mid-pregnancy compared to the virgin gland.
Figure 2. β3 expression is increased in MaSCs during pregnancy.
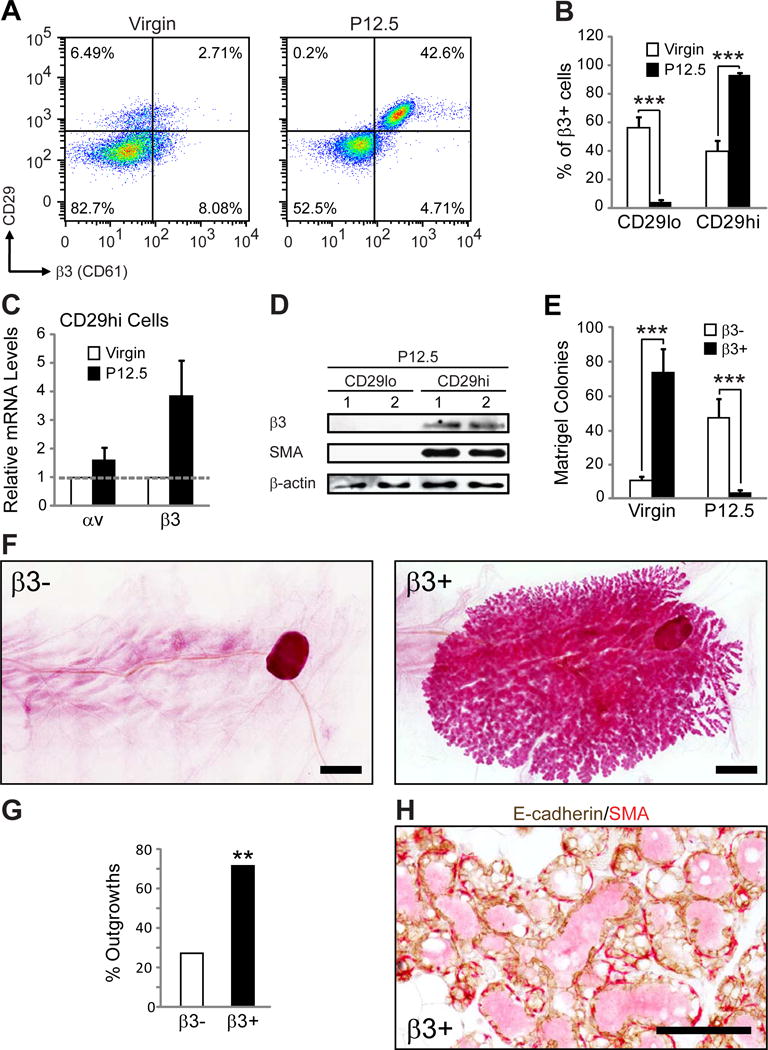
(A,B) FACS analysis of MaSC/progenitor markers in virgin and P12.5 WT mammary glands. (A) Representative FACS density plots showing the live, Lin−CD24+ cells expressed according to their CD29 (β1 integrin) and β3 status. (B) Histograms showing the percent of Lin−CD24+β3+ cells that are CD29lo or CD29hi. P-values for virgin versus P12.5: CD29lo; P=0.00014, CD29hi; P=0.00008. Data shown are mean ± s.e.m. and were analyzed by Student’s T-tests. ***P<0.001. (A,B) Virgin, n=8, P12.5, n=13.
(C) qPCR data showing the relative levels of αv and β3 mRNA in virgin and P12.5 CD29hi cells. Virgin, n=2 (pooled from 2 mice each), P12.5, n=3. Each sample was run in triplicate and 18S rRNA was used as a loading control. Data is displayed as the mean ± s.e.m. fold change (2−ΔΔCT) in P12.5 relative to virgin glands.
(D) Immunoblot of FACS-sorted Lin−CD24+ CD29lo and CD29hi mammary cells from two different P12.5 WT mice for β3, SMA (basal marker) and β-actin (loading control).
(E) Matrigel colonies from live Lin−CD24+β3+ and β3− cells sorted from virgin or P12.5 WT mice. Virgin, n=4, P=0.00003, P12.5, n=4, P=0.0014. Data represent the mean ± s.e.m. and were analyzed by paired Student’s T-tests. ***P<0.001.
(F–H) Mammary gland outgrowth experiments. (F) Representative images of carmine-stained mammary gland outgrowth whole-mounts from P12.5 Lin−CD24+β3+ and β3− donor cells. Recipients were harvested at lactating day 2. Scale bars, 2 mm. (G) Bar graph showing the frequency of successful mammary gland outgrowths from 10,000 Lin−CD24+β3+ and β3− donor cells from P12.5mice. Statistical analysis was performed by Fisher’s exact test. P=0.006. **P<0.01. (H) Representative image of immunohistochemical staining for E-cadherin (brown) and αSMA (red) in sections from Lin−CD24+β3+ cell outgrowths. Scale bar, 100 μm. (F–H) β3−, n=22, β3+, n=21 mammary glands from 3 independent experiments. See also Figure S3.
Consistent with β3 expression in CD29hi MaSC/basal cells during pregnancy we observed that β3+ epithelial cells (Lin−CD24+β3+) from P12.5 mice were unable to form colonies in Matrigel compared to β3− cells (Figure 2E). However, these same cells from virgin mice were enriched for Matrigel colony-forming cells, in agreement with their characterization as luminal progenitors (Asselin-Labat et al., 2007) (Figure 2E). Thus, in addition to differences in CD29 status, β3 expression during pregnancy is associated with a functionally distinct cell population compared to luminal progenitors identified in the virgin mammary gland.
Accordingly, we examined whether β3+ epithelial cells from P12.5 mice were enriched for MaSCs capable of repopulating a fully functional mammary gland similar to CD29hi cells (Asselin-Labat et al., 2010; Shackleton et al., 2006). We tested this possibility by injecting 10,000 Lin−CD24+β3+ and β3− mammary cells from the same donor mice into cleared fat pads of weanling recipients and examining repopulating potential. To simultaneously assess differences in functionality, all outgrowths were harvested at lactating day 2. While few outgrowths were observed in mice injected with β3− cells (27%), β3+ cells were enriched for repopulating potential (71%) (Figure 2F,G), similar to CD29hi cells (Asselin-Labat et al., 2010; Shackleton et al., 2006). Successful outgrowths all appeared swollen with milk (Figure 2F,H) and the presence of both luminal and basal cell types was validated by immunohistochemistry (Figure 2H). These data show that during pregnancy, epithelial expression of β3 is associated with a previously undescribed population of pregnancy-associated MaSCs.
β3 is required for expansion of pregnancy-associated MaSCs
During pregnancy, the proportion of CD29hi MaSC/basal cells increases dramatically compared to CD29lo luminal cells (Figure 2A) resulting in an overall increase in MaSC number compared to the virgin gland (Asselin-Labat et al., 2010). Based on the increased β3 surface expression we observed in P12.5 CD29hi cells, we considered whether β3 is required for expansion of MaSCs at this stage. We observed that loss of β3 from P12.5 CD29hi cells (Figure S4A) decreased the percentage of CD29hi cells relative to CD29lo cells (Figure 3A and Figure S4B). Sorted cell counts showed a similar decrease in the number of CD29hi MaSCs in P12.5 β3KO mice with little or no effect on CD29lo luminal cells (Figure 3B). Interestingly, loss of β3 did not affect the number of either cell type in virgin glands, consistent with the absence of a role for β3 in luminal progenitors or MaSCs at this stage (Figure 3B). These findings support a specific role for β3 in regulating expansion of the MaSC-enriched subset during pregnancy.
Figure 3. β3 is required for MaSC expansion during pregnancy.
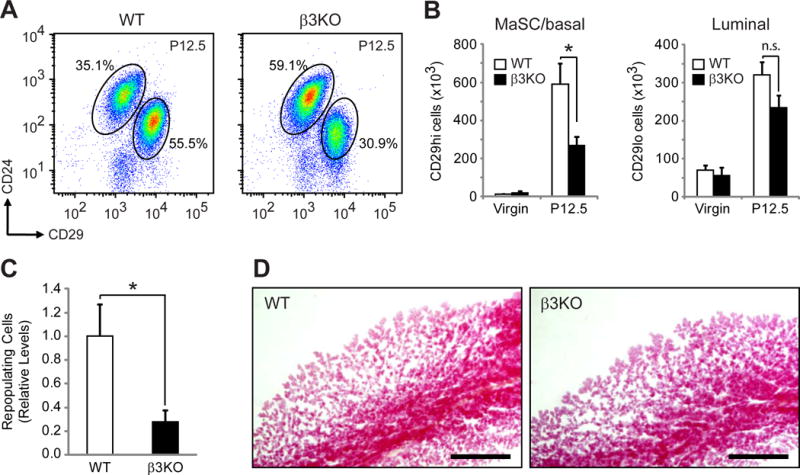
(A,B) FACS analysis of WT and β3KO virgin and P12.5 mammary glands. (A) Representative FACS density plots of WT and β3KO P12.5 mammary cells showing the live, Lin− cells expressed according to their CD24 and CD29 status. (B) Quantitation of the total number of FACS-sorted live Lin−CD24+CD29hi and CD29lo cells from virgin and P12.5 mammary glands. P-values for WT versus β3KO at P12.5: MaSC; P=0.027, Luminal; P=0.09. (A,B) Virgin; WT, n=4, β3KO, n=4, P12.5; WT, n=7, β3KO, n=8.
(C) Histogram showing the relative levels of total repopulating cells in the CD29hi pool from WT and β3KO P12.5 donor mice. n=4 independent experiments. (B,C) Data represent the mean ± s.e.m. and statistical analysis was performed by Student’s T-tests. *P<0.05. (B) n.s. = not significant (P>0.05).
(D) Representative images of carmine-stained WT and β3KO outgrowths harvested at lactating day 2. Scale bars, 1 mm. See also Figure S4.
This defect in CD29hi cell expansion suggests that β3 deletion may result in fewer repopulating cells at mid-pregnancy. To address this we performed limiting dilution mammary gland transplantation assays with CD29hi cells from WT and β3KO P12.5 mammary glands. Results from these experiments showed decreased repopulating frequency in β3KO CD29hi cells (Figure S4C) that corresponded to a 3.6-fold decrease in the absolute number of MaSCs relative to WT mice (Figure 3C). In order to simultaneously test the functionality of these transplants, all outgrowths were harvested on lactating day 2. Interestingly, we noted similar levels of ductal elongation and lobular development in β3KO outgrowths compared to WT (Figure 3D), consistent with the lack of a role for β3 in ductal morphogenesis in the adult gland (Figure 1C) or alveolar maturation during late-pregnancy and lactation (Figures S1D,E and S2A). Accordingly, β3 deletion failed to affect the number of lobule-limited progenitors present within the CD29hi cell pool (Figure S4D). The more limited outgrowths generated by lobule progenitors are defined by the absence of terminal end bud-like ductal extensions preventing invasion of the fat pad (Bruno and Smith, 2011; Jeselsohn et al., 2010; Smith and Medina, 2008) (Figure S4E). Taken together, these observations highlight a critical role for β3 expression in specifically regulating MaSC expansion during mid-pregnancy.
β3 signaling is required for MaSC clonogenicity during pregnancy
Decreased MaSC expansion in pregnant β3KO mice suggests a possible defect in MaSC clonogenicity. To evaluate this role for β3, we examined mammary cells from virgin or P12.5 WT or β3KO mice for colony formation on irradiated mouse embryonic fibroblasts (MEFs). In order to preserve the luminal-basal crosstalk present in the intact mammary gland we used total mammary cells for these experiments. In this assay MaSC and progenitor cells form colonies that can be distinguished based on morphology (Stingl, 2009) (Figure 4A; top panels) and expression of luminal (E-cadherin) or basal (SMA) markers (Jeselsohn et al., 2010) (Figure 4A; bottom panels). Luminal progenitors form densely-packed colonies that express only E-cadherin, basal progenitors form loose colonies that express only SMA, and MaSCs form mixed colonies that express both E-cadherin and SMA (Jeselsohn et al., 2010; Stingl, 2009).
Figure 4. β3 signaling is required for pregnancy-associated MaSC colony formation.
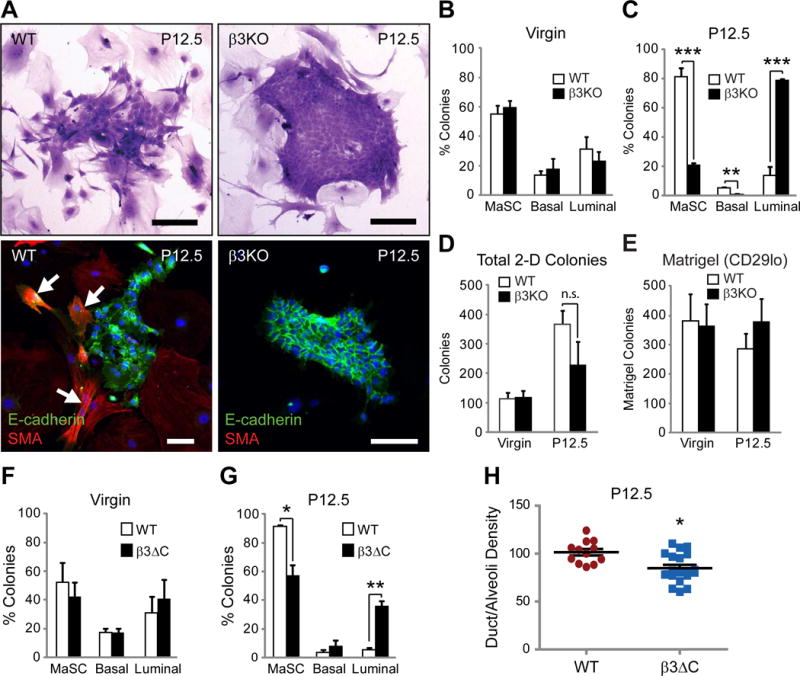
(A) Representative images of WT and β3KO P12.5 colony morphology on irradiated fibroblasts by crystal violet staining (top panels) or immunofluorescent staining for E-cadherin and SMA (bottom panels). Nuclei are stained blue in all panels. Arrows mark SMA-positive cells. Scale bars, 100 μm.
(B–D) Quantitation of the percent MaSC, basal, and luminal colonies (B,C) and total colony number (D) from virgin and P12.5 WT and β3KO mice. Virgin; WT, n=6, β3KO, n=6, P12.5; WT, n=5, β3KO, n=4. (C) P-values for WT versus β3KO at P12.5: MaSC; P=0.0004, Basal; P=0.005, Luminal; P=0.0004. (D) n.s. = not significant (P>0.05).
(E) Histogram depicting colony formation in Matrigel from FACS-sorted CD29lo WT and β3KO cells from virgin and P12.5 mice. Virgin; WT, n=4, β3KO, n=4, P12.5; WT, n=4, β3KO, n=4.
(F,G) MaSC, basal and luminal colonies formed from virgin (F) or P12.5 (G) WT and β3ΔC mammary cells grown on irradiated MEF’s. Virgin; WT, n=2, β3ΔC, n=2, P12.5; WT, n=5, β3ΔC, n=4.
(H) Quantitation of duct/alveoli density in P12.5 WT versus β3ΔC H&E-stained mammary gland sections. WT, n=12, β3ΔC, n=20. (B–H) Data represent the mean ± s.e.m. and statistical analysis performed by Student’s T-tests. *P<0.05, **P<0.01 ***P<0.001. See also Figure S5.
Examining colony morphology by this method we observed that the proportion of MaSC-like mixed colonies increases at P12.5 (Figure 4A; left panels and Figure 4C) compared to virgin glands (Figure 4B), consistent with the increased frequency of MaSCs observed at P12.5 (Asselin-Labat et al., 2010). Unexpectedly, examination of colonies from WT and β3KO mice showed that β3 deletion had no effect on MaSC or luminal colonies in virgin mice, despite β3 acting as a marker of luminal progenitor cells at this stage (Asselin-Labat et al., 2007) (Figure 4B). However, loss of β3 significantly decreased the frequency of MaSC and basal colonies at P12.5 compared to WT mice (Figure 4C), with a reciprocal increase in luminal colonies (Figure 4A; right panels and Figure 4C). Importantly, β3 deletion did not appear to affect total colony number (Figure 4D). The decreased percentage of MaSC colonies in β3KO mice could alternatively be explained by an increase in luminal progenitors. To directly address this possibility, we performed Matrigel colony formation assays with WT and β3KO CD29lo sorted cells from virgin or P12.5 mice and observed no effect of β3 deletion on luminal progenitor cells at either stage (Figure 4E). These data highlight a specific role for β3 in regulating MaSC clonogenicity during pregnancy, while having no effect on luminal progenitors in either the virgin or pregnant mammary gland.
In addition to their role as cell adhesion receptors, integrins activate important signaling pathways influencing a diverse array of cell behaviors including proliferation, survival and migration. To examine a role for β3 signaling in MaSC/progenitor behavior we analyzed knock-in mice expressing a signaling deficient β3 mutant lacking only the last three amino acids of the β3 cytoplasmic domain (β3ΔC) (Ablooglu et al., 2009). This mutant prevents the interaction with c-Src and other signaling proteins (Arias-Salgado et al., 2003), resulting in deficient β3 signaling, but does not influence ligand binding (Ablooglu et al., 2009; Arias-Salgado et al., 2003; Desgrosellier et al., 2009). Importantly, previous studies showed that cells from β3ΔC knock-in mice express similar levels of β3 protein compared to those from WT mice and the β3ΔC mutant forms functional integrin αvβ3 heterodimers capable of mediating adhesion to β3 substrates (Ablooglu et al., 2009), which we validated in P12.5 mammary glands (Figure S5A–D). Similar to the β3KO (Figure 4B,C), no differences were found in colonies from virgin β3ΔC mice (Figure 4F), yet we observed fewer MaSC colonies from β3ΔC mice at P12.5, with proportionally more luminal colonies (Figure 4G). As observed in β3KO mice, total colony number was unchanged upon β3ΔC expression (Figure S5E) and Matrigel assays showed no difference in luminal progenitor cell number (Figure S5F). In fact, examination of P12.5 β3ΔC mammary gland whole-mounts (Figure S5G) and H&E-stained sections (Figure 4H), showed fewer fine branches and alveoli relative to WT mice, similar to the phenotype observed in β3KO mice. These results reveal a role not only for β3 expression, but more specifically the β3 cytoplasmic signaling domain in contributing to P12.5 MaSC clonogenic activation and mammary gland development during pregnancy.
TGFβ2 stimulates β3 expression, enhancing MaSC clonogenicity
While hormones like progesterone have been shown to increase β3 expression in MaSC/basal cells of ovarectomized mice (Joshi et al., 2010), this is unlikely to be a direct affect as MaSCs lack steroid hormone receptors (Asselin-Labat et al., 2006). Therefore, to investigate the factors that may account for β3 expression in MaSCs during pregnancy, we evaluated several paracrine factors associated with pregnancy such as Transforming Growth Factor β (TGFβ) family members and Receptor Activator of NF-κB Ligand (RANKL). Importantly, both RANKL and TGFβ ligands are increased during pregnancy (Asselin-Labat et al., 2010; Fata et al., 2000; Monks, 2007; Robinson et al., 1991), and both pathways affect development of the pregnant mammary gland (Fata et al., 2000; Gorska et al., 2003). Further, RANKL and TGFβ ligands are known to regulate β3 expression in other systems (Galliher and Schiemann, 2006; Lacey et al., 1998).
To examine the ability of these factors to increase β3 expression, we stimulated cells from virgin WT mice and measured β3 expression in MaSC/basal cells, defined by expression of K14 and SMA. Unexpectedly, we found that TGFβ2, but not TGFβ1 or RANKL, stimulated β3 expression in MaSC/basal cells relative to vehicle-treated cells (Figure 5A). QPCR analysis confirmed TGFβ2’s ability to drive β3 mRNA expression specifically in CD29hi MaSC/basal cells as little effect was observed in CD29lo luminal cells from the same mice (Figure 5B,C). Similar effects were observed in human mammary epithelial cells where TGFβ2 was a potent driver of β3 expression (Figure 5D). Indeed, we found that TGFβ2 was capable of directly activating the β3 promoter in these cells as determined using a luciferase reporter plasmid containing the proximal 1300 base pairs of the β3 promoter upstream of the initial start site (Figure 5E). While TGFβ family members commonly induce activation of the SMAD family of transcription factors, analysis of the β3 promoter failed to identify any SMAD consensus binding elements as assessed by querying the ENCODE whole-genome data in the UCSC genome browser (Raney et al., 2011). However, SMADs commonly exert their transcriptional effects through interacting with SP1 (Feng et al., 2000; Jungert et al., 2006; Poncelet and Schnaper, 2001), which has previously been shown to directly bind the β3 promoter (Evellin et al., 2013). Accordingly, knock-down of SP1 potently blocked TGFβ2-induced β3 mRNA and protein expression (Figure 5F,G).
Figure 5. TGFβ2 stimulates β3 expression, enhancing MaSC clonogenicity.
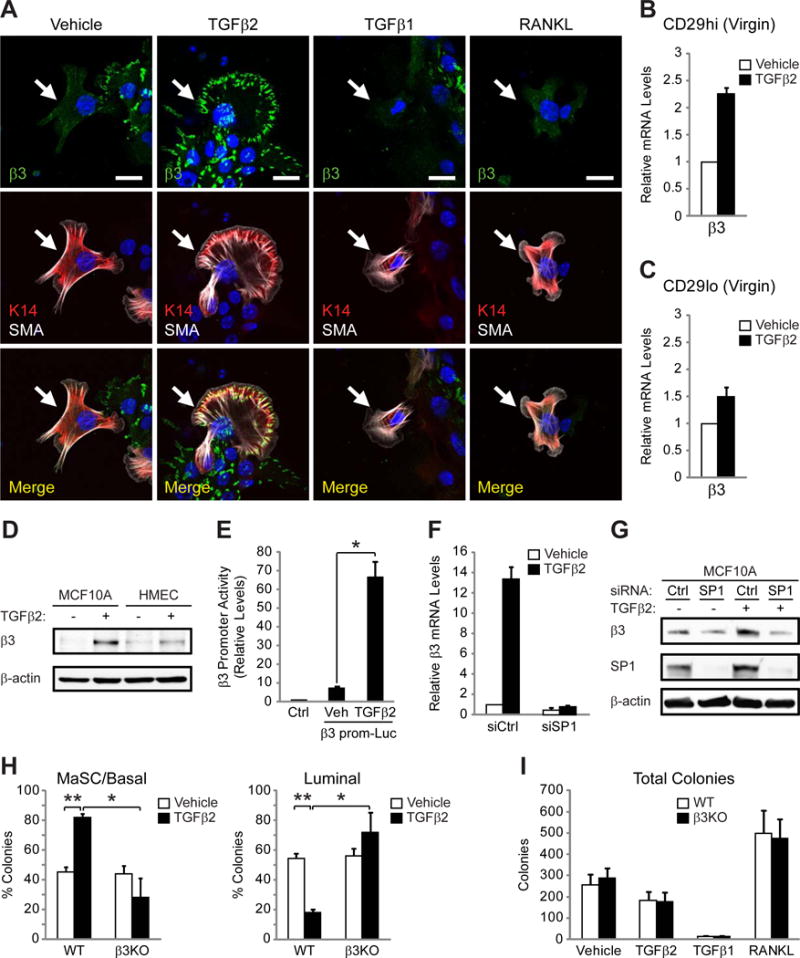
(A) Representative immunofluorescent images of β3 expression in K14+SMA+ cells (arrows) from pooled virgin WT mammary cells stimulated with the indicated growth factors. Nuclei are stained blue in all panels. Scale bars, 20 μm. Data shown are representative of 3 independent experiments.
(B,C) qPCR analysis comparing the relative levels of β3 mRNA in vehicle versus TGFβ2-stimulated CD29hi (B) and CD29lo (C) cells from WT virgin mice. n=2 independent experiments (pooled samples).
(D) Immunoblot for β3 and β-actin (loading control) in MCF10As and human mammary epithelial cells (HMECs) stimulated with TGFβ2 or vehicle control. Data shown is representative of 3 independent experiments.
(E) Histogram displaying the relative luciferase activity in MCF10A cells transfected with an empty vector (Ctrl) or a luciferase reporter plasm id containing the proximal region of the β3 promoter (β3 prom-Luc) and stimulated with vehicle or TGFβ2. n=3 independent experiments. P=0.043.
(F,G) A representative experiment showing the effect of SP1 knock-down on β3 mRNA (F) and protein (G) expression in MCF10A cells stimulated with TGFβ2 or vehicle control. MCF10A cells transfected with control (siCtrl) or SP1 siRNA (siSP1) were analyzed for β3 mRNA expression by qPCR (F) or β3 protein by immunoblot (G) in the same experiment. n=3 independent experiments. (B,C,F) Each sample was run in triplicate and 18S rRNA (B,C) or β-actin (F) were used as loading controls. Data is displayed as the mean ± s.e.m. fold change (2−ΔΔCT).
(H,I) Quantitation of the percent MaSC/basal, and luminal colonies (H) and total colony number (I) from pooled virgin WT and β3KO mammary cells stimulated with the indicated growth factors. n=3 independent experiments. (H) P-values for vehicle versus TGFβ2 in WT cells: MaSC; P=0.0071, Luminal; P=0.0071, and WT versus β3KO cells stimulated with TGFβ2: MaSC; P=0.0413, Luminal; P=0.0413. (E,H,I) Data represent the mean ± s.e.m. and statistical analysis performed by Student’s T-tests. *P<0.05, **P<0.01.
The ability of TGFβ2 to drive β3 expression suggested that this ligand may affect MaSC clonogenicity in a β3-dependent manner. Compared to vehicle, only TGFβ2 increased the frequency of bipotent, MaSC-like colonies grown on irradiated MEFs (Figure 5H), with a reciprocal decrease in luminal colonies (Figure 5H), an effect similar to that observed in cells from pregnant mice (Figure 4B,C). This effect was reduced in mammary cells from β3KO mice (Figure 5H), similar to our results from pregnant β3KO mice (Figure 4C). TGFβ2 did not affect total colony number unlike TGFβ1, which had a severe growth inhibitory effect, and RANKL which acted as a potent driver of colony formation (Figure 5I). Notably, β3 did not contribute to colony number in response to any of these factors consistent with results from P12.5 β3KO mice (Figure 4D). Taken together, these data show that TGFβ2, a critical growth factor released during pregnancy, stimulates β3 expression in MaSC/basal cells and enhances MaSC clonogenicity in a β3-dependent manner.
β3 mediates Slug activation in response to TGFβ2 and pregnancy
The bipotent MaSC-like colonies observed in response to TGFβ2 possess morphological characteristics reminiscent of an epithelial-mesenchymal transformation (EMT) (Figure 5H). This is consistent with a relationship between EMT genes and MaSC/progenitor cell behavior (Guo et al., 2012), particularly during pregnancy (Chakrabarti et al., 2012). Additionally, TGFβ family members are well-characterized for their ability to stimulate EMT in development (Moustakas and Heldin, 2007) and cancer (Katsuno et al., 2013). Consistent with this, TGFβ2 stimulation of MCF10A cells drives changes in the expression of EMT markers such as Slug, E-cadherin and Vimentin (Figure S6A). While TGFβ2 induced similar effects on EMT marker protein expression in WT, but not β3KO 2-D colonies from virgin mice (Figure S6B,C), no such changes in mRNA expression were noted in sorted CD29hi or CD29lo cells (Figure S6D). Thus, TGFβ2-stimulated changes in colony morphology (Figure 5H) are consistent with increased formation of MaSC-like bipotent colonies, and not an EMT transition of either luminal or basal cells types.
Despite the apparent absence of an effect on EMT, Slug protein levels were consistently reduced in TGFβ2-stimulated β3KO 2-D colonies compared to WT, specifically in the K14+SMA+ MaSC/basal cells (Figure S6C). Slug was recently characterized as a determinant of the MaSC fate in the virgin mammary gland (Guo et al., 2012) and is expressed during early to mid-pregnancy, but is negatively regulated by Elf5 during late-stage pregnancy/lactation allowing for alveolar maturation (Chakrabarti et al., 2012). Therefore, we considered whether β3 was required for TGFβ2-mediated Slug expression in MaSC/basal cells. In virgin WT mammary cells, TGFβ2 induced nuclear Slug expression specifically in K14+SMA+ cells compared to cells treated with a vehicle control (Figure 6A,B). In contrast, TGFβ2 failed to increase Slug in β3KO cells (Figure 6A,B) highlighting an essential role for β3 in TGFβ2-stimulated Slug protein expression in MaSC-enriched basal cells, despite the absence of an effect on Slug m RNA (Figure S6D). Given that TGFβ2 expression is induced during pregnancy (Robinson et al., 1991), we hypothesized that β3 may similarly regulate Slug expression in the pregnant mammary gland. Compared to WT glands, we observed dramatically reduced levels of nuclear Slug in the K14+ basal cells of P12.5 β3KO mice (Figure 6C,D). Despite this decrease in Slug protein levels, Slug mRNA was unaffected in β3KO P12.5 CD29hi cells (Figure S6E) similar to the effects observed in TGFβ2-stimulated cells (Figure S6D,F). Thus, β3 appears to play an integral role in regulating Slug protein expression in MaSC-enriched basal cells in response to TGFβ2 or pregnancy with no effect observed on Slug mRNA.
Figure 6. β3 is required for Slug activation in response to TGFβ2 or pregnancy.
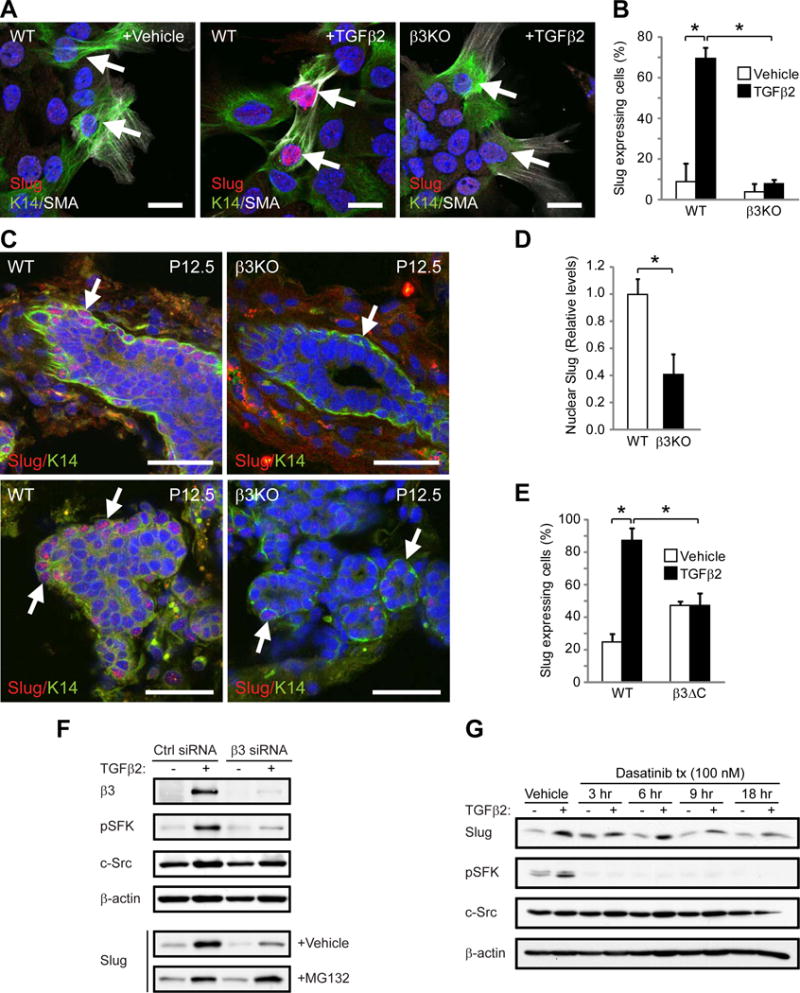
(A,B) Slug expression in K14+SMA+ cells from virgin WT and β3KO mammary cells stimulated with vehicle or TGFβ2. (A) Representative images of Slug expression in K14+SMA+ cells (arrows). Scale bars, 20 μm. (B) Quantitation of the percentage of Slug+K14+SMA+ cells. P=0.0439 (vehicle versus TGFβ2 in WT cells) and P=0.0342 (WT versus β3KO cells stimulated with TGFβ2). (A,B) WT, n=3, β3KO, n=3.
(C,D) Slug expression in WT and β3KO P12.5 mammary glands. (C) Representative images of Slug in K14+ cells (arrows). Scale bars, 20 μm. (A,C) Nuclei are stained blue in all panels. (D) Histogram showing the relative levels of nuclear Slug expression. Data for each mouse represents the average nuclear Slug expression from 5 fields normalized to total nuclear stain. P=0.0113. (C,D) WT, n=8, β3KO, n=6.
(E) Quantitation of the percentage of Slug-expressing K14+SMA+ cells from virgin WT and β3ΔC mammary cells stimulated with vehicle or TGFβ2. WT, n=2, β3ΔC, n=2. P=0.029 (vehicle versus TGFβ2 in WT cells) and P=0.032 (WT versus β3ΔC cells stimulated with TGFβ2). (B,D,E) Data represent the mean ± s.e.m. and statistical analysis performed by Student’s T-tests. *P<0.05.
(F,G) Immunoblots of MCF10A cells stimulated with TGFβ2 or vehicle control and probed for the indicated proteins. (F) Cells were transfected with Control (Ctrl) or β3 siRNA and additionally treated with vehicle or proteasome inhibitor (MG132) for 5 hr prior to lysis. (G) Cells were treated with 100 nM Src inhibitor (Dasatinib) for the indicated length of time prior to lysis. (F,G) Data shown is representative of 3 independent experiments. See also Figures S6 and S7.
To determine the potential mechanism by which αvβ3 regulates Slug we considered whether αvβ3 signaling may be required by assessing whether the β3ΔβC mutant effects Slug expression. Whereas TGFβ2 induced Slug in K14+SMA+ mammary cells from virgin WT mice, no increase was observed in cells from β3ΔC knock-in mice (Figure 6E). The β3ΔC mutant has previously been characterized as defective in recruiting and activating Src family kinases (SFKs) (Ablooglu et al., 2009; Arias-Salgado et al., 2003; Desgrosellier et al., 2009). Consistent with this, we noted decreased levels of SFK activation (pY416 SFK) in TGFβ2-stimulated K14+SMA+ MaSC/basal cells from β3ΔC virgin mice compared to WT cells (Figure S7A) suggesting that αvβ3-mediated SFK activation may be required for Slug expression. Indeed, transient β3 knock-down in MCF10A cells significantly reduced TGFβ2-induced pY416 SFK and Slug expression (Figure 6F) with no effect observed on Slug mRNA (Figure S6F). Interestingly, treatment with an αvβ3 function-blocking antibody (LM609) failed to effect SFK activation or Slug expression (Figure S7B), suggesting that this role for αvβ3 may be ligand-independent. The absence of an effect on Slug mRNA suggested that αvβ3 may regulate Slug protein through an alternative mechanism. Indeed, Slug protein levels are highly regulated through degradation by the proteasome (Kim et al., 2012; Wu et al., 2012). We found that short-term incubation with a proteasome inhibitor (MG132) was sufficient to restore Slug protein levels to normal in β3 knock-down cells with little effect on Slug levels in control cells (Figure 6F). Accordingly, short-term incubation with the SFK inhibitor Dasatinib reduced only the TGFβ2-induced Slug protein expression (Figure 6G). Thus, it appears that TGFβ2-stimulated expression of αvβ3 mediates SFK activation resulting in enhanced Slug protein stability.
αvβ3 is associated with Slug activation and stemness in human breast cancer cells
Previous studies showed that Slug promotes properties associated with both MaSCs and aggressive stem-like breast cancer cells (Guo et al., 2012; Proia et al., 2011). Our observation that αvβ3 regulates Slug in pregnancy-associated MaSCs prompted us to investigate whether a similar relationship exists in human breast cancer cells. Indeed, using gain- and loss-of-function approaches (Figure 7A–C and S7C–F) we found that ectopic expression of β3 was sufficient to drive Slug nuclear accumulation in both MCF-7 and MDA-MB-468 human breast cancer cells, which lack endogenous β3 (Figure 7A,C and S7D,F) while β3 shRNA knock-down in a highly metastatic (HM) variant of the MDA-MB-231 cells (Munoz et al., 2006) reduced nuclear Slug levels compared to control cells expressing a non-silencing shRNA (Figure 7B and S7E). Notably, even unligated αvβ3 was capable of driving Slug expression as assessed with a β3 mutant deficient in ligand binding (β3 D119A) (Desgrosellier et al., 2009) (Figure S7C). This is consistent with the inability of αvβ3 antagonists to inhibit Slug expression in non-transformed cells (Figure S7B) and suggests a ligand-independent role for αvβ3. Additionally, the β3ΔC mutant was defective in Slug expression compared to full-length β3 (Figure 7C and S7F) in agreement with our observations from mice (Figure 6E). Thus, β3 is both necessary and sufficient for Slug activity in human breast cancer cells in addition to regulating Slug expression in pregnancy-associated MaSCs in the mouse.
Figure 7. αvβ3 is associated with Slug activation and stemness in human breast cancer cells.
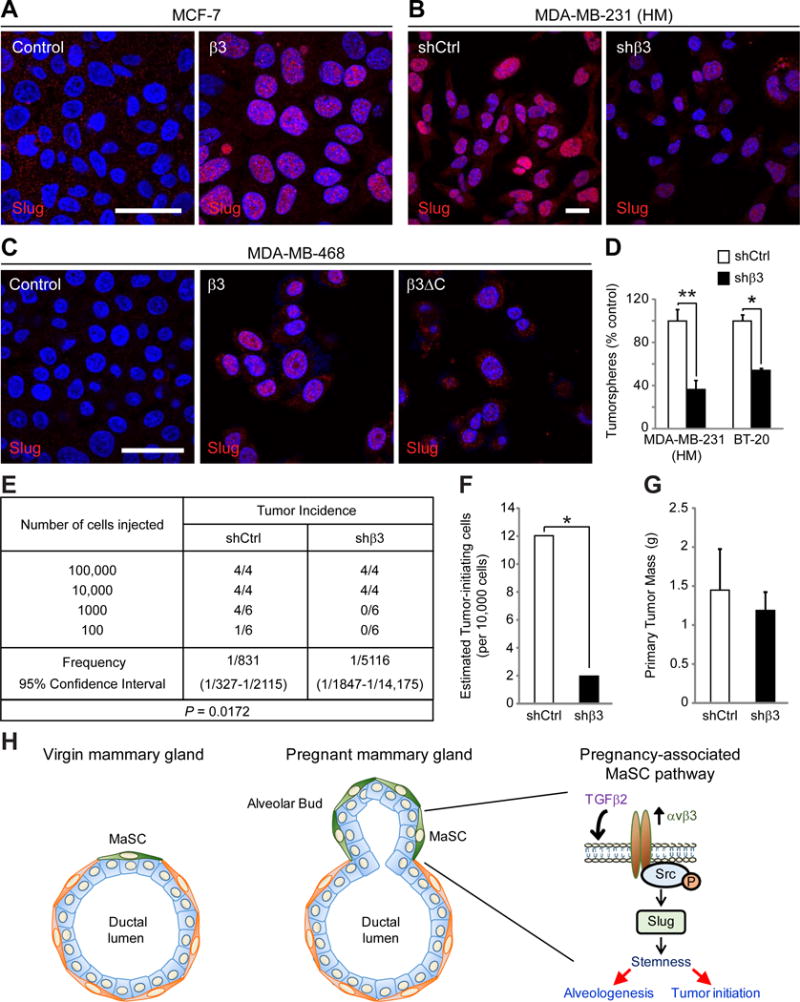
(A–C) Representative immunofluorescent images showing Slug expression in (A) MCF-7 cells stably transfected with β3 cDNA or vector alone (Control) (B) a highly metastatic (HM) variant of MDA-MB-231 cells stably expressing a non-silencing (shCtrl) or β3 shRNA (shβ3) and (C) MDA-MB-468 cells stably expressing vector control, full-length β3 or the β3ΔC mutant. (A–C) Nuclei are stained blue in all panels. Scale bars 20 μm.
(D) Histogram depicting the results of β3 knock-down on soft agar colony number in MDA-MB-231 (HM) or BT-20 human tumor cell lines compared to control. MDA-MB-231 (HM), n=3, P=0.0079, BT-20, n=2, P=0.031.
(E–G) In vivo tumor initiation studies comparing control and β3 knock-down MDA-MB-231 (HM) cells injected orthotopically into adult female mice at limiting dilution. (E) Table describing the frequency of tumor formation per fat pad injected for each cell type. (F) Histogram showing the estimated number of tumor-initiating cells from the data in (E). (G) Bar graph depicting the primary tumor mass for each cell type in tumors formed after injection of 10,000 cells and harvested at 6 weeks. (D,G) Data represent the mean ± s.e.m. and statistical analysis performed by Student’s T-test. *P<0.05, **P<0.01.
(H) Schematic describing the function of the αvβ3-Src-Slug signaling axis in MaSC expansion during pregnancy. Compared to the virgin mammary gland (left panel) pregnancy induces expansion of the MaSC population (green cells), resulting in the initiation of alveologenesis (middle panel). Factors released during pregnancy, such as TGFβ2, drive αvβ3 expression in these pregnancy-associated MaSCs, resulting in activation of Src family kinases and increased levels of Slug (right panel). This pathway may lead not only to MaSC expansion and alveologenesis during pregnancy, but may additionally contribute to stem-like properties in breast cancer cells, resulting in tumor initiation. See also Figure S7.
These findings suggest that αvβ3 may promote stem-like properties in tumor cells which we assessed by tumorsphere formation in vitro and limiting dilution tumor initiation experiments in vivo. Consistent with the role of unligated αvβ3 in promoting Slug expression, stable β3 knock-down in triple negative BT-20 and MDA-MB-231 (HM) cells resulted in fewer anchorage-independent tumorspheres relative to controls (Figure 7D and S7E,G). Additionally, β3 knockdown in MDA-MB-231 (HM) cells reduced the number of tumor-initiating cells compared to control when injected orthotopically into adult female mice at limiting dilution (Figure 7E,F). Importantly, β3 knock-down had no effect on primary tumor mass in these experiments (Figure 7G), indicating that αvβ3 has a specific effect on tumor-initiating cells and does not affect basic proliferative and survival responses necessary for primary tumor growth, similar to the effects observed in the mammary gland due to β3 deletion. Together, our findings highlight a conserved role for αvβ3 leading to Slug activity associated with MaSC expansion during pregnancy and stem-like properties in breast cancers.
DISCUSSION
Integrin αvβ3 is found in some of the most aggressive tumor cells in a diverse array of carcinomas including breast cancer, where it is associated with enhanced tumorigenicity and metastasis (Desgrosellier et al., 2009; Felding-Habermann et al., 2001; Liapis et al., 1996; Sloan et al., 2006; Takayama et al., 2005) yet it is unclear if this is related to a role in epithelial stem/progenitor cells. We now show that αvβ3 plays a specific role in driving MaSC expansion during pregnancy. Genetic deletion of the integrin β3 subunit, or expression of a signaling deficient form of this receptor, resulted in defective mammary gland development during pregnancy with no affect on ductal morphogenesis in the virgin gland. This phenotype was associated with increased expression of β3 in the MaSC-enriched pool at mid-pregnancy, an effect reproduced by stimulation with TGFβ2 (Figure 7H). Examination of MaSC and progenitor cell activity showed that β3 was specifically required for MaSC clonogenicity, expansion and Slug expression during pregnancy with no effect on luminal progenitor cells.
Distinct MaSC/progenitor populations contribute to the development, maintenance and remodeling of the adult mammary gland (Asselin-Labat et al., 2010; Spike et al., 2012; Van Keymeulen et al., 2011; Wagner et al., 2002). MaSC behavior is highly sensitive to steroid hormones released during the estrus cycle and pregnancy (Asselin-Labat et al., 2007; Asselin-Labat et al., 2010; Joshi et al., 2010) and these pregnancy-induced MaSCs possess distinct properties compared to MaSCs in the virgin gland such as more limited self-renewal (Asselin-Labat et al., 2010). Additionally, some MaSC/progenitors are retained post-pregnancy in the parous gland where they represent a functionally distinct population of parity-induced cells (Matulka et al., 2007). We observed that β3 levels associated with MaSCs during pregnancy were transient, diminishing to levels found in virgin mice after involution. Thus, β3-expressing MaSCs are unlikely to represent parity-induced cells. Instead, our findings characterize integrin αvβ3 as a critical determinant of the MaSC state during mid-pregnancy and a requirement for αvβ3 serves to distinguish these cells from MaSCs required for maintenance in the virgin gland.
To our surprise β3 was not required for luminal progenitor cell function despite characterization of β3 as a surface marker of luminal progenitor cells in the virgin mammary gland (Asselin-Labat et al., 2007). While our findings show that β3 enriches for luminal progenitors in the virgin gland, consistent with others (Asselin-Labat et al., 2007), genetic deletion of β3 had no effect on luminal progenitor cell clonogenicity or ductal morphogenesis. This is consistent with other reports where genetic deletion of β3 had no effect on ductal morphogenesis in virgin adult murine mammary glands (Taverna et al., 2005). Thus, a potential role for αvβ3 in tumor cell clonogenicity may be linked to its expression on MaSCs during pregnancy rather than on luminal progenitors.
The steroid hormone progesterone is critical for mammary gland remodeling during pregnancy and regulates β3 expression in MaSCs (Joshi et al., 2010). However, MaSCs lack the progesterone receptor (Asselin-Labat et al., 2006), suggesting that progesterone regulates MaSCs indirectly through stimulating release of paracrine factors such as TGFβ and RANKL during pregnancy (Asselin-Labat et al., 2010; Fata et al., 2000; Monks, 2007; Robinson et al., 1991). We show that the TGFβ family member TGFβ2, and not TGFβ1 or RANKL, drives β3 expression in MaSC/basal cells enhancing MaSC clonogenicity. Similar to our observations regarding β3 expression and function during pregnancy, progesterone and TGFβ family members are critically expressed early in pregnancy (Gorska et al., 2003; Jhappan et al., 1993; Monks, 2007; Robinson et al., 1991) and are reduced in late-pregnancy, allowing lobular maturation (Gorska et al., 2003; Jhappan et al., 1993; Monks, 2007; Robinson et al., 1991). Thus, integrin αvβ3 may function as a key molecular switch downstream of progesterone-TGFβ signaling that promotes the activation of the MaSC pool during early pregnancy and is reduced in late pregnancy allowing for alveolar secretory maturation.
Pregnancy-associated breast cancers represent some of the most aggressive breast cancers due to frequent metastasis (Schedin, 2006). Interestingly, recent studies have shown that pregnancy is a major regulator of MaSC number and function, suggesting a relationship between MaSCs and pregnancy-associated breast cancers (Asselin-Labat et al., 2010). Accordingly, some proteins that regulate MaSCs during pregnancy also have important functions in aggressive breast tumors (Gonzalez-Suarez et al., 2010; Schramek et al., 2010). Our findings reveal a specific role for integrin αvβ3 in regulating Slug expression and MaSC expansion during pregnancy. In breast cancer cells, αvβ3 also appears to be necessary and sufficient for Slug activity, anchorage-independent growth, and tumor initiation, properties of stem-like cancer cells (Figure 7H). These findings highlight a potential relationship between αvβ3’s function in pregnancy-associated MaSCs and aggressive stem-like breast cancers.
EXPERIMENTAL PROCEDURES
Histological analysis, immunohistochemistry and immunofluorescence
For immunohistochemical staining of formalin-fixed paraffin-embedded tissues, antigen retrieval was performed in citrate buffer at pH 6.0 and 95°C for 20 min. Sections were blocked in normal goat serum diluted in PBS, incubated overnight at 4°C in primary antibody followed by biotin-conjugated anti-rabbit IgG and an avidin–biotin peroxidase detection system with 3,3′-diaminobenzidine substrate (Vector) then counterstained with hematoxylin. Whole-mount mouse mammary glands were fixed in Carnoy’s solution and stained with carmine. For quantitation of duct/alveoli density, 3–4 images were randomly sampled from H&E-stained paraffin sections from each mouse with a 4× objective and analyzed with Metamorph software. For immunofluorescence, frozen sections or fixed cells were blocked with normal goat serum in PBS and incubated in primary antibody overnight at 4°C followed by secondary at room temperature for 1 h. For further details, see Supplemental Experimental Procedures.
Lysates and immunoblotting
Whole mammary gland lysates were prepared by pulverizing glands flash frozen in liquid nitrogen with a mortar and pestle and then lysing the tissue with RIPA lysis buffer. The lysate was further processed with a hand-held tissue homogenizer and cleared. Whole cell lysates were prepared from cell lines with RIPA lysis buffer combined with scraping. Standard Western blotting procedures were performed. See Supplemental Experimental Procedures for further details.
Flow cytometry and mammary outgrowth assays
Single cell suspensions were prepared and stained with antibodies as described in detail in Supplemental Experimental Procedures. Cell sorting was performed using a FACS Diva or FACS Aria (BD). For outgrowth experiments, sorted cells were injected into the cleared abdominal fat pads of 3 week old syngeneic recipients. Estimated repopulating cell frequencies were calculated using the ELDA web-based tool (Hu and Smyth, 2009) (http://bioinf.wehi.edu.au/software/elda/).
Mammary colony assays
For colony formation on irradiated MEF’s, 100,000 MEF’s were seeded into 6-well dishes for 48 hr prior to adding 40,000 cells from digested mammary glands and grown in complete DMEM medium. Colonies formed over 5–6 days before fixing and staining with either 0.1% crystal violet/20% methanol/PBS or 2% paraformaldehyde/PBS for immunofluorescent staining and counting colonies.
For Matrigel colonies, 5,000 FACS-sorted mammary gland cells were suspended in 50 μl growth-factor-reduced Matrigel (BD Pharmingen) and grown 14 days in serum-free Mammary Epithelial Cell Medium-Basal Medium (Cambrex) supplemented with B27 supplement, 20 ng ml−1 epidermal growth factor, 20 ng ml−1 bFGF, 4 μg ml−1 heparin, 100 units ml−1 penicillin, 100 μg ml−1 streptomycin. Total colonies per well were counted from each of the 4 replicates per experiment.
Growth factor and inhibitor experiments
Cells from digested mammary glands were seeded at 40,000 cells per well onto MEF’s (for colony formation assays) or 8-well chamberslides (Lab-Tek) coated with 2% Matrigel/DMEM. At the time of seeding, cells were suspended in complete DMEM medium supplemented with vehicle (0.1% BSA/PBS), RANKL (50 ng ml−1), TGFβ1 (5 ng ml−1) or TGFβ2 (5 ng ml−1) (Peprotech). Cells were fixed with 2% paraformaldehyde/PBS after 48 h (chamberslides) or 5 days (colonies) for immunofluorescent staining.
For experiments with MCF10A and HMECs, TGFβ2 stimulations were performed with 5 ng ml−1 TGFβ2 (Peprotech) or 0.1% BSA/PBS (vehicle) for 48 h prior to lysis. In some experiments 30 ng ml−1 of the anti-αvβ3 function blocking antibody LM609 (Millipore) was added to cells at the same time as TGFβ2 or vehicle addition. For the proteasome inhibitor experiments, 10 μM MG132 (Sigma) or DMSO (vehicle) was added to transfected MCF10A cells 5 h prior to lysis. Treatment of MCF10A cells with the Src family kinase inhibitor Dasatinib (Chemietek) or DMSO (vehicle) was performed with 100 nM Dasatinib for the indicated times prior to harvesting lysates.
Orthotopic breast cancer
Tumors were generated by injection of MDA-MB-231 (HM) cells expressing non-silencing or β3 shRNA at limiting dilution (in 50 μL sterile PBS) into the inguinal fat pads of adult (12 weeks) female NSG mice. Mice were monitored weekly for tumor formation by gentle palpation. Primary tumor mass was determined by assessing the wet weight of the resected tumors. All tumors formed within 5 weeks, and all tumor-bearing mice were harvested at 6 weeks. Tumor-free mice were harvested at 13 weeks and the absence of any detectable tumor confirmed by whole-mount staining.
Statistical analyses
Data presentation and statistical tests are indicated in the figure legends. For all analyses, P<0.05 was considered statistically significant.
Supplementary Material
Highlights.
Pregnancy increases expression of the integrin αvβ3 in mammary stem cells (MaSCs)
αvβ3 is critical for initiating alveologenesis and remodeling during pregnancy
αvβ3 mediates pregnancy-induced MaSC expansion, clonogenicity and Slug expression
In breast cancer, αvβ3 similarly regulates Slug expression and stem-like behavior
Acknowledgments
We would like to thank Brandon Grosshart and Fernanda Camargo for their technical assistance as well as Hisashi Kato for generously providing lentiviral plasmids containing the β3 and β3ΔC cDNAs. We would also like to acknowledge the histology and flow cytometry shared resource facilities at the Moores UCSD Cancer Center. This work was supported by US National Institutes of Health grants CA168692, HL57900 and R3750286 (to D.A.C.) and funding from the California Breast Cancer Research Program grant number 18IB-0020 (to J.S.D.). S.K. was supported by the National Cancer Institute of the National Institutes of Health award number T32CA121938.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.S.D designed the project, performed experiments, analyzed the data and wrote the manuscript. J.L., L.S., M.G., S.K., A.F., and M.Y. helped design and conduct experiments and analyze data. S.J.S. provided help and expertise related to the β3ΔC mouse experiments. D.A.C. analyzed the data, supervised the overall project and wrote the manuscript.
References
- Ablooglu AJ, Kang J, Petrich BG, Ginsberg MH, Shattil SJ. Antithrombotic effects of targeting alphaIIbbeta3 signaling in platelets. Blood. 2009;113:3585–3592. doi: 10.1182/blood-2008-09-180687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98:1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- Bai L, Rohrschneider LR. s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev. 2010;24:1882–1892. doi: 10.1101/gad.1932810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RD, Smith GH. Functional characterization of stem cell activity in the mouse mammary gland. Stem Cell Rev. 2011;7:238–247. doi: 10.1007/s12015-010-9191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Hwang J, Andres Blanco M, Wei Y, Lukacisin M, Romano RA, Smalley K, Liu S, Yang Q, Ibrahim T, et al. Elf5 inhibits the epithelial-mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat Cell Biol. 2012;14:1212–1222. doi: 10.1038/ncb2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15:1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evellin S, Galvagni F, Zippo A, Neri F, Orlandini M, Incarnato D, Dettori D, Neubauer S, Kessler H, Wagner EF, et al. FOSL1 controls the assembly of endothelial cells into capillary tubes by direct repression of alphav and beta3 integrin transcription. Molecular and cellular biology. 2013;33:1198–1209. doi: 10.1128/MCB.01054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Lin X, Derynck R. Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15(Ink4B) transcription in response to TGF-beta. The EMBO journal. 2000;19:5178–5193. doi: 10.1093/emboj/19.19.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- Gorska AE, Jensen RA, Shyr Y, Aakre ME, Bhowmick NA, Moses HL. Transgenic mice expressing a dominant-negative mutant type II transforming growth factor-beta receptor exhibit impaired mammary development and enhanced mammary tumor formation. Am J Pathol. 2003;163:1539–1549. doi: 10.1016/s0002-9440(10)63510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C, Hinds PW. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17:65–76. doi: 10.1016/j.ccr.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhappan C, Geiser AG, Kordon EC, Bagheri D, Hennighausen L, Roberts AB, Smith GH, Merlino G. Targeting expression of a transforming growth factor beta 1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. The EMBO journal. 1993;12:1835–1845. doi: 10.1002/j.1460-2075.1993.tb05832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- Jungert K, Buck A, Buchholz M, Wagner M, Adler G, Gress TM, Ellenrieder V. Smad-Sp1 complexes mediate TGFbeta-induced early transcription of oncogenic Smad7 in pancreatic cancer cells. Carcinogenesis. 2006;27:2392–2401. doi: 10.1093/carcin/bgl078. [DOI] [PubMed] [Google Scholar]
- Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim YM, Yang CH, Cho SK, Lee JW, Cho M. Functional regulation of Slug/Snail2 is dependent on GSK-3beta-mediated phosphorylation. The FEBS journal. 2012;279:2929–2939. doi: 10.1111/j.1742-4658.2012.08674.x. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Liapis H, Flath A, Kitazawa S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol. 1996;5:127–135. doi: 10.1097/00019606-199606000-00008. [DOI] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F, Yagita H, Lindeman GJ, Smyth GK, Visvader JE. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12:R21. doi: 10.1186/bcr2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Monks J. TGFbeta as a potential mediator of progesterone action in the mammary gland of pregnancy. J Mammary Gland Biol Neoplasia. 2007;12:249–257. doi: 10.1007/s10911-007-9056-2. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, Kerbel RS. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Poncelet AC, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells. The Journal of biological chemistry. 2001;276:6983–6992. doi: 10.1074/jbc.M006442200. [DOI] [PubMed] [Google Scholar]
- Proia TA, Keller PJ, Gupta PB, Klebba I, Jones AD, Sedic M, Gilmore H, Tung N, Naber SP, Schnitt S, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell stem cell. 2011;8:149–163. doi: 10.1016/j.stem.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raney BJ, Cline MS, Rosenbloom KR, Dreszer TR, Learned K, Barber GP, Meyer LR, Sloan CA, Malladi VS, Roskin KM, et al. ENCODE whole-genome data in the UCSC genome browser (2011 update) Nucleic acids research. 2011;39:D871–875. doi: 10.1093/nar/gkq1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SD, Silberstein GB, Roberts AB, Flanders KC, Daniel CW. Regulated expression and growth inhibitory effects of transforming growth factor-beta isoforms in mouse mammary gland development. Development. 1991;113:867–878. doi: 10.1242/dev.113.3.867. [DOI] [PubMed] [Google Scholar]
- Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GH, Medina D. Re-evaluation of mammary stem cell biology based on in vivo transplantation. Breast Cancer Res. 2008;10:203. doi: 10.1186/bcr1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike BT, Engle DD, Lin JC, Cheung SK, La J, Wahl GM. A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell stem cell. 2012;10:183–197. doi: 10.1016/j.stem.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J. Detection and analysis of mammary gland stem cells. J Pathol. 2009;217:229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res. 2005;25:79–83. [PubMed] [Google Scholar]
- Taverna D, Crowley D, Connolly M, Bronson RT, Hynes RO. A direct test of potential roles for beta3 and beta5 integrins in growth and metastasis of murine mammary carcinomas. Cancer Res. 2005;65:10324–10329. doi: 10.1158/0008-5472.CAN-04-4098. [DOI] [PubMed] [Google Scholar]
- Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell stem cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG, Weiss SJ. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci U S A. 2012;109:16654–16659. doi: 10.1073/pnas.1205822109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


