Abstract
Objectives
To evaluate the histologic characteristics of paranasal sinus mucosa of a disease control population and children with chronic rhinosinusitis and cystic fibrosis (CRS/CF) (1) to determine whether goblet cell (GC) hyperplasia and/or submucosal gland (SMG) hyperplasia occur in pediatric CRS/CF and (2) to compare expression and localization ofMUC5ACand MUC5B mucins in the sinus mucosa of both cohorts.
Design
Histologic and morphometric analyses of paranasal sinus mucosa were used to quantify the number of GCs and mucin-expressing cells. Digital imaging was used to evaluate the SMG area. Immunohistochemistry was performed to identify the cellular localization of MUC5AC and MUC5B mucins, and confocal microscopy was used to determine whether MUC5AC and MUC5B mucins were expressed in the same secretory cells.
Setting
Children’s National Medical Center, Washington, DC.
Participants
Twenty-one children with CRS/CF who underwent endoscopic sinus surgical procedures and 18 children who underwent craniofacial resection or neurosurgical procedures for abnormalities other than sinusitis.
Results
A statistically significant increased area (4.4-fold) of SMGs was detected in the sinus mucosa of patients with CRS/CF compared with the controls (P = .02). Neither GC hyperplasia nor increased expression of MUC5AC was observed in the CRS/CF group. MUC5AC was expressed only in a subpopulation of GCs in both cohorts, and MUC5B was expressed in a subpopulation of GCs as well as in SMGs. There was a positive trend toward increased glandular MUC5B expression in the CRS/CF cohort. Colocalization of MUC5AC and MUC5B expression was observed in a subset of GCs.
Conclusions
Significant SMG hyperplasia and a trend toward increased glandular MUC5B expression exist in children with CRS/CF. This suggests that SMG hyperplasia and glandular MUC5B mucin contribute to mucus overproduction in the sinus mucosa of this population.
Cystic fibrosis (CF) is a multiorgan obstructive exocrinopathy characterized by chronic inflammation, recurrent infections, and abnormal respiratory tract mucus secretions.1 Many patients with CF develop chronic rhinosinusitis (CRS); these patients (CRS/CF) often exhibit nasal polyposis and chronic mucus hypersecretion/overproduction and obstruction, which alters their sinonasal mucociliary clearance patterns.2–4 Mucus hypersecretion/ overproduction in respiratory tract diseases is the culmination of several complex processes, including hyperplasia of mucin-secreting goblet cells (GCs) in the conducting epithelium and/or hyperplasia of submucosal glands (SMGs).5 Goblet cell and/or SMG hyperplasia can be age, disease, and/or tissue specific. For example, both GC and SMG hyperplasia are more prevalent with age in the lower respiratory tract tissues of patients with CF.6,7 In contrast, adult and pediatric patients with CRS exhibit SMG hyperplasia8,9 but not GC hyperplasia8,10 in their sinus mucosa. It is not known whether patients with CRS/CF express the same phenotype in their sinus mucosa as those with just CRS.
Mucins are the major macromolecular components of mucus and account for its rheologic properties.11,12 Mucins are characterized by tandem repeats in their protein backbones and are encoded by a specific human MUC gene. MUC5B and MUC5AC, 2 of the 18 mucins identified to date, are the major mucins expressed in the respiratory tract.5 MUC5AC is usually restricted to GCs in the lower13 and upper10 respiratory tracts. MUC5B, typically restricted to SMGs in the lungs of both disease controls and patients with CF,14 is expressed not only in SMGs but also in GCs in the sinus mucosa of disease control patients and patients with CRS.9 In addition, MUC5B is reported to be increased in sinus mucosal secretions from adults with CRS15 and in nasal polyps from adults with CF,16 suggesting a role for MUC5B in the upper respiratory tract of adults with CRS or CRS/CF.
Patients with CF often develop CRS,2,3 and the mucus hypersecretion and obstruction associated with CRS/CF may be complicated by alterations in mucin expression or localization. However, to our knowledge, there is no literature describing the expression or distribution of mucins in the sinonasal tissues of children or adults with CRS/CF, highlighting the need for further research. Altered mucin expression in the sinus mucosa of patients with CRS/CF vs those with CRS or disease controls would suggest that this alteration contributes to the mucus hypersecretion/obstruction observed in patients with CRS/CF. The objectives of this study were to evaluate the histologic characteristics of paranasal sinus mucosa of a disease control population and children with CRS/CF and determine whether GC hyperplasia and/or SMG hyperplasia occur with CRS/CF and to compare expression and localization of MUC5AC and MUC5B mucins in the sinus mucosa of both cohorts.
METHODS
PATIENT POPULATION
Tissue samples were obtained from patients with CRS/CF who underwent an endoscopic sinus surgical procedure (ESS) for CRS refractory to medical management. All patients were classified as children (<21 years) by National Institutes of Health criteria (http://grants.nih.gov/grants/guide/notice-files/not98-024.html). Cystic fibrosis was confirmed by sweat test and/or genetic evaluation for the CFTR gene (OMIM *602421). Chronic rhinosinusitis was defined as the presence of symptoms for more than 3 months, despite antimicrobial and topical nasal corticosteroid therapies, and included at least 2 of the major signs and symptoms: nasal congestion, rhinorrhea, headache, facial pain/pressure, nasal obstruction, or change in olfaction.17,18 All patients in the CRS/CF cohort underwent nasal endoscopy before ESS and received antibiotics and/or topical nasal corticosteroids at the time of the operation. Pulmonologists evaluated the children with CRS/CF before surgery. Patients with ciliary dyskinesias or craniofacial abnormalities were excluded. Computed tomography scans of the sinuses were obtained, evaluated, and scored by means of the Lund-Mackay system17 in the CRS/CF group; all scans received a minimum score of 8.
Sinus tissues from patients who underwent craniofacial and/or neurosurgical procedures for abnormalities other than sinusitis served as disease controls. Exclusion criteria for the disease controls included a history of sinonasal operations, current sinonasal infection, sinonasal or allergic symptoms within the preceding 3 months, and/or treatment with topical nasal corticosteroids within 1 month or antihistamines within 3 months before the operation. A combination of computed tomography scans and/or magnetic resonance images with sinonasal sections was obtained. There was no radiographic evidence of sinusitis at the time of the operation for children in the disease control group.10 All patients were entered consecutively into the study after appropriate surgical and research consents (and assents when applicable) were obtained. This study was reviewed and approved by the institutional review board of Children’s National Medical Center. There was no disease control group for CF without CRS because the institutional review board cannot give approval for obtaining sinus mucosa from patients with CF without CRS that is recalcitrant to medical management.
SPECIMEN COLLECTION, PROCESSING, AND HISTOCHEMICAL STAINING
By means of standard endoscopic techniques, directed biopsies were taken from the right and left paranasal sinuses at the time of ESS, based on medical necessity. All patients with CRS/CF had sinus tissues collected from either the maxillary or ethmoid sinuses in the vicinity of the osteomeatal complex. Whenever possible, sinus tissues from the disease controls were also collected as close as possible to the osteomeatal complex. Either maxillary and/or anterior ethmoid sinus mucosa was evaluated in 14 of 18 disease controls. In 2 disease controls, sphenoid sinus mucosa was analyzed. Data on the specific sinus entered were not recorded for 2 of the disease controls.
Tissues were fixed in 10% neutral buffered formalin (pH, 6.8–7.2; Richard-Allan Scientific, Kalamazoo, Michigan) for paraffin embedding and sectioning. Tissue sections (5 µm) were cut in the Division of Pathology at Children’s National Medical Center. Each slide was stained with hematoxylin and eosin to evaluate tissue quality.
IMMUNOCHEMISTRY
Protocols were adapted from previous studies performed on patients with CRS and children used as disease controls at Children’s National Medical Center.9,10 Briefly, paraffin sections were de-paraffinized and endogenous peroxidase activity was blocked using hydrogen peroxide in methyl alcohol (30 minutes). Sections were incubated with a MUC5AC mouse monoclonal antibody (1:300; Lab Vision Corporation, Fremont, California) and aMUC5Brabbit polyclonal antibody (1:300, sc-20119; Santa Cruz Biotechnology, Inc, Santa Cruz, California) overnight at 4°C. After phosphate-buffered saline washes, sections were incubated with biotinylated horse antimouse or horse antirabbit antibody (1:250; Vector Laboratories, Burlingame, California) for 1 hour. Secondary antibody was detected with a stain (Vectastain ABC Elite Kit; Vector Laboratories) and 3′ 3′ diaminobenzidine substrate (Sigma-Aldrich Co, St Louis, Missouri). Slides were counterstained with hematoxylin (Sigma-Aldrich Co) and mounted. In double-staining experiments with Alcian blue/periodic acid–Schiff (AB/PAS), before counterstaining with hematoxylin, slides were stained with Alcian blue (30 minutes), rinsed, and treated with periodic acid (10 minutes) and Schiff reagent (5 minutes). Slides were then counterstained with hematoxylin and mounted. Omission of primary antibody was a negative control. Human stomach tissue was a positive control for MUC5AC expression,19 and salivary tissue was a positive control for MUC5B expression.20
QUANTITATIVE ANALYSES OF SUBMUCOSAL GLANDS
Three randomly selected fields of AB/PAS-stained tissues in each specimen were analyzed. Under ×200 magnification, fields were captured from each section with a charger-coupled device camera-assisted microscope (Nikon Eclipse E800; Nikon, Tokyo, Japan) and the images were analyzed (Image J; http://rsb.info.nih.gov/ij). The ratio between the area of AB/PAS-stained glands and total area of tissue was recorded. Data were presented as the mean of 3 fields for each specimen.
A grading classification of MUC5B staining in SMGs was developed to account for variable MUC5B staining in each specimen and allow comparison between the 2 groups under investigation. Grade 1 was considered an area of MUC5B-positive staining that was 50% or less of the total SMG area per high-power field (×400); grade 2, an area of MUC5B-positive staining that was more than 50% but less than 100% of the total SMG area; and grade 3, 100% of the glandular area was positive for MUC5B staining. Two observers (M.M.A. and M.T.P.) masked to the patients’ clinical profile and type of mucosal sample independently evaluated the slides.
QUANTIFICATION OF EPITHELIAL CELLS
Specimens were coded and the number of AB/PAS-positive GCs and MUC5AC- or MUC5B-immunoreactive cells in the epithelial layer of the sinus mucosa in 10 high-power field was counted by 2 observers (M.M.A. and M.T.P.) (Olympus light microscope BH2; Olympus, Tokyo). A 1-cm2 grid reticle divided into 100 square units was used to quantify cell number per millimeter of epithelium. The length of each unit was measured to be 0.25 mm of tissue at a magnification of ×400.21 Cells in 4 of these units (0.25 mm each) were counted to determine the number of GCs and MUC5AC/MUC5B staining cells per millimeter of tissue. Only sections with distinct, intact, positively staining cells containing nuclei were counted.
STATISTICAL ANALYSES
To quantitatively evaluate differences in SMGs in CRS/CF and disease control specimens, data were analyzed by a nonpaired 2-sample t test, assuming nonequal variances. The closeness of the independent counts by 2 observers for the numbers of epithelial cells, mucin-positive cells in the epithelium, and glandular MUC5B-positive cells was analyzed to determine their intraclass coefficient correlation by a Pearson correlation program (SPSS Inc, Chicago, Illinois). Statistical analyses were performed using commercial software (SPSS and Microsoft Excel 2003; Microsoft Corporation, Redmond, Washington).
MUCIN LOCALIZATION BY MULTICHANNEL FLUORESCENCE MICROSCOPY
Deparaffinized and rehydrated slides were placed in 10 mM sodium citrate buffer (pH, 6.0) and boiled for 10 minutes at 95°C for antigen unmasking. For dual staining, tissues were incubated with mouse anti-MUC5AC primary monoclonal antibody (1:200; Lab Vision Corporation) and rabbit anti-MUC5B primary polyclonal antibody (1:100, sc-20119; Santa Cruz Biotechnology, Inc) overnight at 4°C and then with the secondary fluorescent-labeled antibodies Alexa Fluor 488 goat antimouse IgG (1:250, A11004; Invitrogen, Carlsbad, California) and Alexa Fluor 568 goat antirabbit IgG (1:250, A11004; Invitrogen) for 1 hour at room temperature. Sections were mounted (ProLong Gold antifade reagent, P36934; Invitrogen) with a cover glass and examined using a microscope (Zeiss LSM 510 confocal microscope; Carl Zeiss Microimaging, Thornwood, New York). For negative controls, the primary or secondary antibody was omitted. Images were taken at magnifications of ×200 and ×630 under oil immersion.
RESULTS
Sinus mucosa samples from 21 patients with CRS/CF (age, 66–252 months; mean, 241 months) and 18 disease controls (age, 120–238 months; mean, 191 months) were studied.
HISTOCHEMICAL ANALYSES OF SINUS MUCOSA
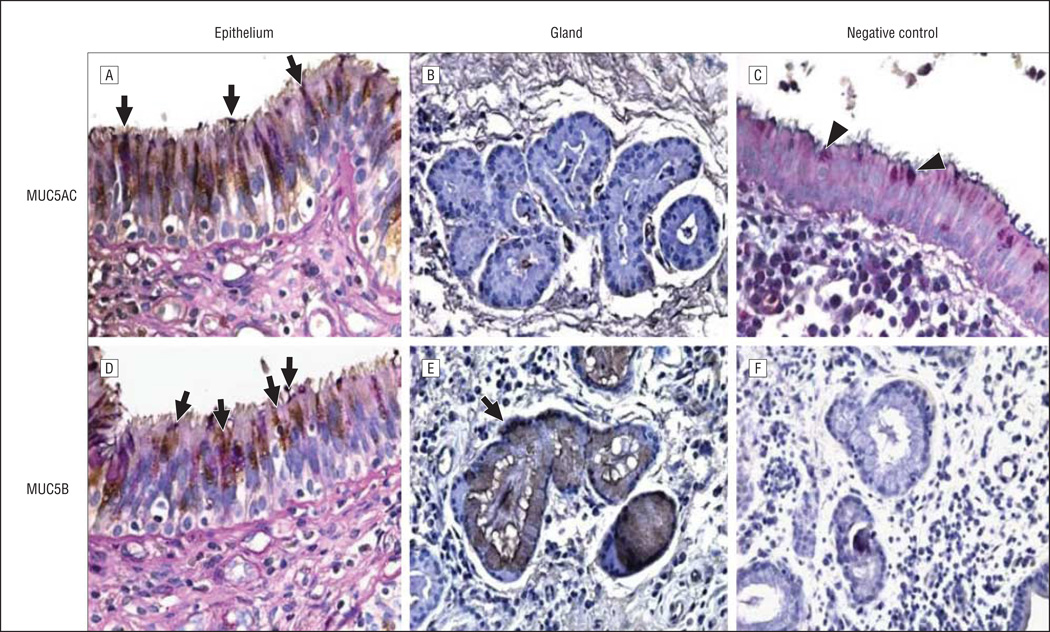
The surface epithelial and submucosal microanatomical compartments from 8 CRS/CF and 8 disease control sinus mucosal specimens were evaluated histologically. Goblet cells and mucous cells in SMGs were identified by AB/PAS staining.22 Staining was evaluated in the intact epithelium and glands (when present) of both cohorts. Figure 1 shows typical AB/PAS-positive cells in the surface epithelium of sinus mucosa from disease control (Figure 1A) and CRS/CF (Figure 1D) samples, demonstrating the presence of GCs in both cohorts. Submucosal glands were typically sparse in the sinus mucosa of disease control specimens but could be identified (Figure 1B); they were abundant in CRS/CF sinus mucosa (Figure 1E).
Figure 1.
Immunostaining for MUC5AC and MUC5B in the sinus mucosa; Alcian blue/periodic acid–Schiff (AB/PAS) and hematoxylin counterstaining was then applied. Disease controls (A and B), chronic rhinosinusitis/cystic fibrosis tissues (D and E), and negative controls (C and F) are shown. All goblet cells (GCs) are stained with AB/PAS purple/blue stain (A and D, arrows; and C, arrowheads). Brown/purple staining shows GCs that co-stain with MUC5AC (A, arrows). There was no staining for MUC5AC in the glands (B). Brown/purple staining shows GCs (D, arrows) and submucosal glands (E, arrow) that co-stain with MUC5B. Original magnification ×600 for epithelia (A, C, and D), ×400 for glands (B, E, and F).
COMPARISON OF SMG AREA IN DISEASE CONTROL AND CRS/CF SINUS MUCOSA
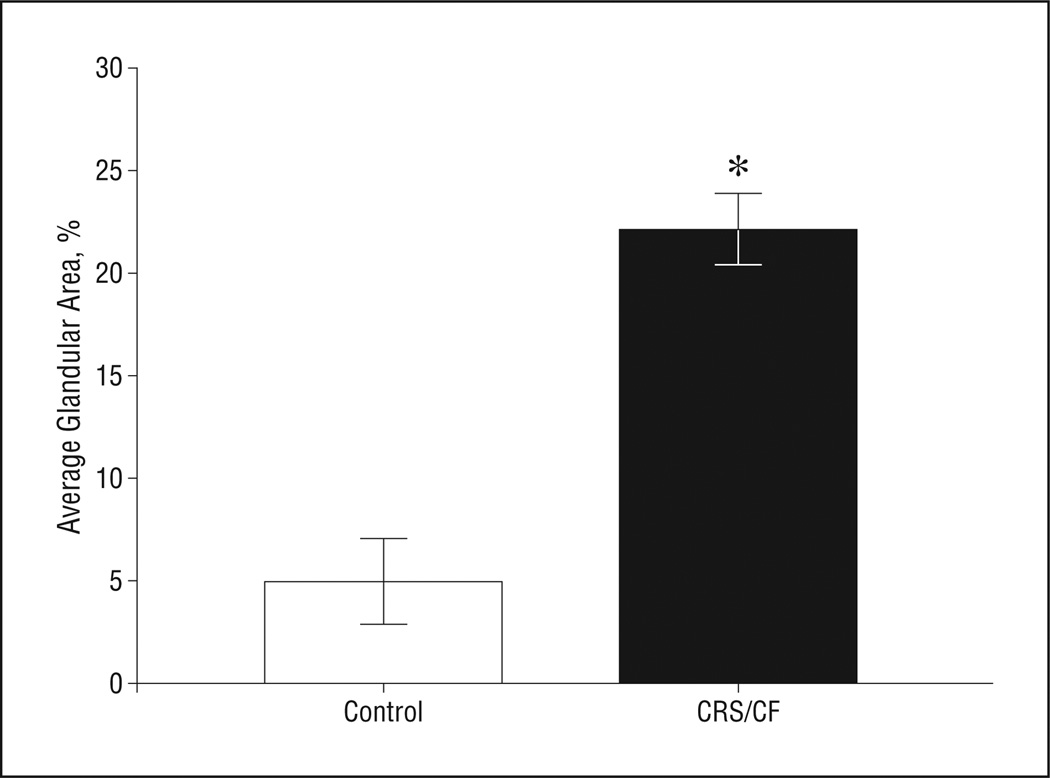
Differences in the area of SMGs in the CRS/CF and disease control populations were determined by image analysis for 15 specimens (6 disease control samples that contained SMGs and 9 with CRS/CF). The data demonstrated that the area of the glands was significantly increased 4.4-fold in samples from patients with CRS/CF compared with those from disease controls (Figure 2) (P = .02).
Figure 2.
Quantification of submucosal gland (SMG) area in the sinus mucosa. The ratio of SMG area to total tissue area was averaged for disease control (n = 6) and for chronic rhinosinusitis/cystic fibrosis (CRS/CF) specimens (n = 9) and shown as a bar graph. *A statistically significant increase of SMG area in CRS/CF sinus mucosa was observed (t test, P = .02). Limit lines indicate SE.
LOCALIZATION OF MUCIN EXPRESSION IN THE SINUS MUCOSA
Sinus mucosal samples from 8 of 21 patients with CRS/CF and 8 of 18 disease controls were used for immunohistochemical analysis of both MUC5AC and MUC5B. MUC5AC mucin was not expressed in SMGs of disease control samples where glands were identified (Figure 1B). Similar patterns were observed with mucosal specimens from patients with CRS/CF (data not shown). MUC5B expression, which was clearly detected in all SMGs in CRS/CF samples (Figure 1E) and in disease control samples (data not shown), was evaluated by a grading classification. Of 8 CRS/CF specimens, 75.0% had grade 2 (n = 4) or grade 3 (n = 2) MUC5B expression and 2 were scored as grade 1. Normal tissues (n = 6) were distributed evenly among all 3 grades. However, not all glandular cells or all SMGs in either the CRS/CF or disease control population expressed MUC5B. In independent masked analysis, 2 observers (M.M.A. and M.T.P.) were in 100% agreement on grading assessments. The data suggested a trend toward increased expression of MUC5B in the SMGs of CRS/CF specimens compared with disease control specimens.
Immunohistochemical analyses of tissue samples from 5 patients with CRS/CF and 8 disease controls were performed to determine the cellular localization of MUC5AC mucin protein in sinus tissues. Representative micrographs of MUC5AC expression in sinus mucosal specimens from disease controls are shown (Figure 1). MUC5AC-positive cells were localized exclusively to the epithelial compartment of the sinus mucosa (Figure 1A) and were not detected in SMGs (Figure 1B). Similar patterns were observed with mucosal specimens from patients with CRS/CF (data not shown). To confirm that the MUC5AC-positive cells were GCs, tissues were immunostained with anti-MUC5AC antibody and subsequently stained with AB/PAS. MUC5AC expression was localized to GCs for both the disease control and CRS/CF groups, but not all GCs expressed MUC5AC mucin. To determine whether GCs in the sinus mucosa of patients with CRS/CF expressed MUC5B mucin, similar analyses were performed. MUC5B-positive cells were well expressed in a subset of GCs in the sinus mucosa in both the disease control (data not shown) and CRS/CF groups (Figure 1D).
QUANTIFICATION OF GCS AND MUC5AC/MUC5B-POSITIVE CELLS
To determine whether there was increased GC hyperplasia or increased MUC5AC or MUC5B expression in the epithelial layer of CRS/CF sinus mucosa, specimens from both cohorts were reviewed. Sinus mucosa from 8 disease controls were used for MUC5AC analyses, but only 5 of 8 CRS/CF specimens used for immunohistochemical analysis had sufficiently well-preserved epithelium for MUC5AC quantitative studies. Five samples from 4 patients with CRS/CF and 4 from 4 disease control patients were used for MUC5B analyses. The ratio of MUC-positive cells to AB/PAS-positive cells per millimeter of sinus mucosal epithelium is summarized in Table 1 and Table 2. There were no significant differences in the number of GCs staining for either MUC5AC or MUC5B between the CRS/CF and disease control groups and no significant differences between the number of GCs in the disease control vs the CRS/CF cohorts. However, considerable variability with regard to GCs in CRS/CF specimens was noted. Denuded segments of epithelium were often seen in the CRS/CF samples, and there were regions in the epithelium layer of the mucosa where the majority of GCs expressed either MUC5AC or MUC5B.
Table 1.
Quantification of Goblet Cells and MUC5AC-Positive Cells in Sinus Mucosa From Patients With CRS/CF and Disease Control Patient
| Mean (SD) | |||
|---|---|---|---|
| Type of Measurement | Disease Controls |
CRS/CF |
P Valuea |
| MUC5AC-positive cells, cells/mm | 4.89 (0.73) | 5.5 (1.12) | .32 |
| Goblet cells, cells/mm | 9.48 (0.38) | 8.88 (0.90) | .25 |
| MUC5AC/goblet cells | 0.51 (0.06) | 0.6 (0.09) | .21 |
Abbreviation: CRS/CF, chronic rhinosinusitis/cystic fibrosis.
By nonpaired 2-sample t test analysis.
Table 2.
Quantification of Goblet Cells and MUC5B-Positive Cells in Sinus Mucosa From Patients With CRS/CF and Disease Control Patient
| Mean (SD) | |||
|---|---|---|---|
| Type of Measurement | Disease Controls |
CRS/CF |
p Valuea |
| MUC5B-positive cells, cells/mm | 5.59 (1.60) | 5.31 (1.60) | .40 |
| Goblet cells, cells/ mm | 10.23 (1.21) | 9.52 (0.68) | .15 |
| MUC5B/goblet cells | 0.54 (0.14) | 0.55 (0.15) | .45 |
Abbreviation: CRS/CF, chronic rhinosinusitis/cystic fibrosis.
By nonpaired 2-sample t test analysis.
There was a significant correlation between the cell counts by the 2 different analysts for MUC5AC expression (P < .001; Pearson correlation, 0.93), MUC5B expression (P = .04; Pearson correlation, 0.69), AB/PAS GCs stained in the MUC5AC group (P = .001; Pearson correlation, 0.78), and AB/PAS-positive cells between both groups (P < .001; Pearson correlation, 0.79). The only group that showed a weak correlation was the AB/PAS GCs counted in the MUC5B group in CRS/CF patients (Pearson correlation, 0.01).
COLOCALIZATION OF MUC5AC AND MUC5B MUCINS IN GOBLET CELLS
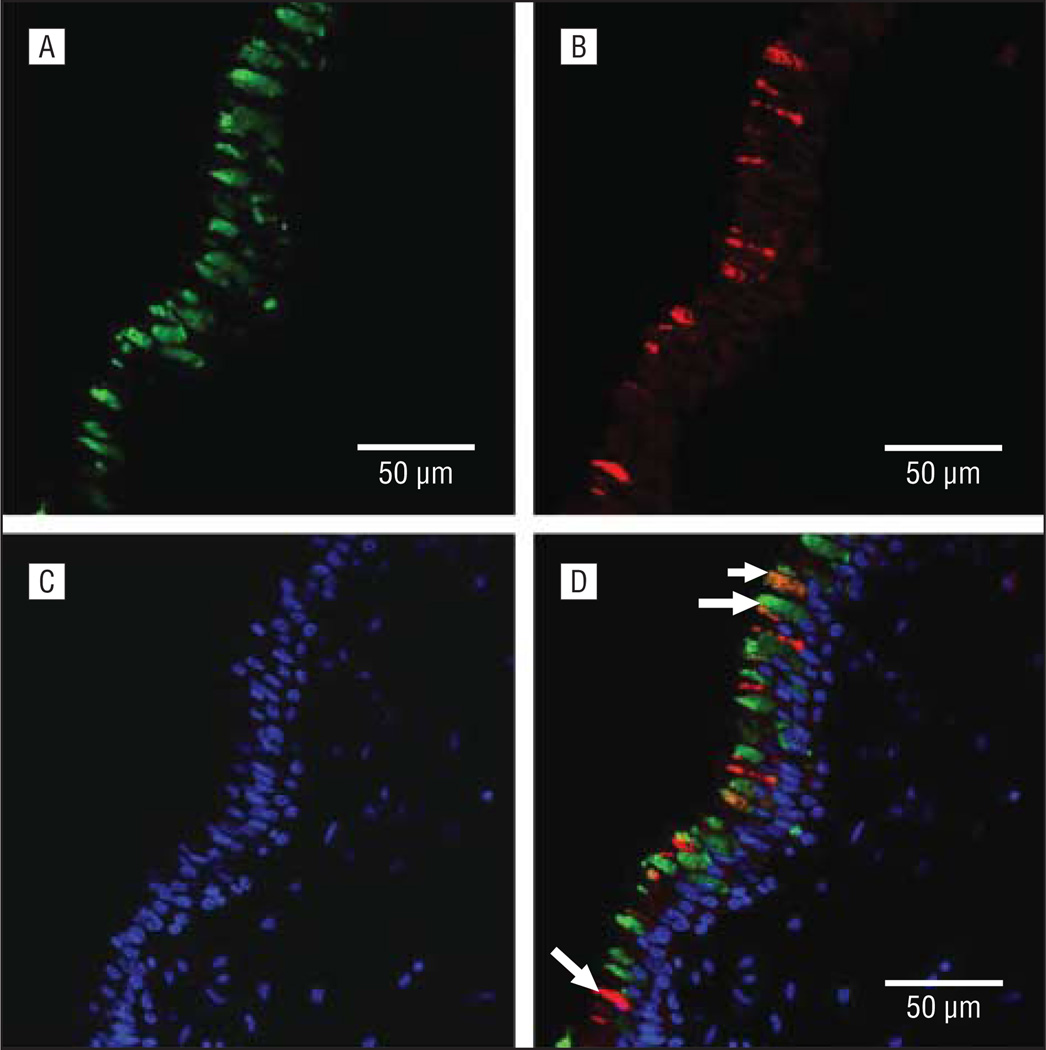
To determine whether MUC5AC and MUC5B mucins were expressed in the same GCs or in different subsets, double immunofluorescent staining and multichannel fluorescence microscopy were used on sinus tissues from 4 patients with CRS/CF and 4 disease controls. Colocalization studies demonstrated the presence of (1) a subset of GCs that expressed only MUC5AC (Figure 3A), (2) a subset of GCs that expressed only MUC5B (Figure 3B), and (3) a small subset of GCs in which MUC5AC and MUC5B mucins were colocalized (Figure 3D). There were no apparent differences in the colocalization and distribution of either MUC5AC or MUC5B in the sinus epithelium of the disease control and CRS/CF specimens.
Figure 3.
Representative micrographs of immunofluorescent double staining of MUC5AC and MUC5B mucins in the sinus mucosa. Images of MUC5AC (A), MUC5B (B), and nuclear marker 4′, 6-diamidino-2-phenylindole (DAPI) (C) are shown separately, then merged (D); individual staining for MUC5AC or MUC5B in separate cells (long arrows) and colocalization of MUC5AC and MUC5B in a single cell (short arrow) shown in part D.
COMMENT
Mucus overproduction/hypersecretion is related to the pathophysiologic characteristics of obstructive diseases in the upper (CRS, CRS/CF) and lower (CF) respiratory tracts. Cystic fibrosis is characterized by chronic inflammation and infection and overproduction of highly viscous mucus that obstructs the respiratory tracts. Mutations in CFTR are responsible for the clinical manifestations of CF, but it remains unclear how they translate into various phenotypic presentations.1,4 Recent studies23 have suggested that other genes, including those involved in airway defense, ion transport, and lipid metabolism, may play a significant role in determining the clinical presentation of each patient with CF. Chronic rhinosinusitis, on the other hand, is characterized by poor ciliary function and edema, inflammation, and stagnant mucus in the sinus mucosa that may reflect dysfunctional host immune responses.24 Chronic rhinosinusitis is often manifested in CF, with 20% of patients developing nasal polyposis and most becoming infected with bacterial pathogens in their upper respiratory tract.4 Therapy for patients with CRS/CF is particularly challenging, both medically and surgically, because of their recalcitrant sinus disease and nasal polyposis.2,25
Mucin expression in nasal or sinus mucosal tissues of patients with CF, CRS, or other upper airway diseases has recently been reviewed9,10,26,27; to our knowledge, this is the first study on mucin expression in CRS/CF sinus mucosa. The data demonstrated that MUC5AC and MUC5B mucins were expressed in GCs in the sinus epithelium of both the CRS/CF and disease control cohorts and that the numbers of MUC5AC- or MUC5B-expressing GCs were similar in the study groups, demonstrating a lack of GC hyperplasia in the sinus mucosa of patients with CRS/CF. These results are similar to those reported9,10 earlier for children with CRS but without CF, indicating that the pathophysiologic characteristics of CRS are present in the sinus mucosa epithelium of patients with CRS/CF, even though the etiologic characteristics of the diseases differ. Interestingly, most GCs in the sinus mucosa of both cohorts expressed only MUC5AC or MUC5B, in contrast to a recent study28 in lower respiratory tract tissues showing that MUC5B is typically expressed in GCs that manifest extensive colocalization of MUC5 Band MUC5AC in people who smoke and have chronic obstructive pulmonary disease and GC hyperplasia.
Patients with CRS/CF clearly exhibited SMG hyperplasia, which is a characteristic phenotype of both children and adults with CRS.9 Our data also showed a trend in the expression of MUC5B expression in SMGs of patients with CRS/CF. Since MUC5B is the major mucin expressed by SMGs, these data suggest that glandular MUC5B secreted by the increased number of SMGs may be the major component of the hypersecreted, stagnant, and viscous mucus observed in patients with CRS, including those with concomitant CF. This is in agreement with data in the literature15 demonstrating that MUC5B levels are higher in the sinus mucosa of patients with CRS than in disease controls, although it is not clear whether the sinus mucus specimens are secretions or sinus mucosa.
Biochemical or biophysical information on mucus secretions from patients with CRS or CRS/CF is almost nonexistent. Clinically, it appears that the physical properties of mucus from these patients are different, with CRS/CF mucus being more viscous. It is very likely that the complex and poorly understood relationship between inflammation, bacterial and viral pathogens, and patient comorbidities affect the quality and viscosity of sinonasal secretions in these diseases, but these pathways have yet to be elucidated. In addition to the suspected increased levels of MUC5B in CRS and CRS/CF, differences in mucus properties could be accounted for by expression of mucin genes not yet evaluated and/or other mucosal proteins or mucins with altered glycosylation patterns in the sinus mucosa of these patients. Biochemical analyses of mucins in the sinonasal secretions from patients with CRS/CF or CRS and healthy individuals would clarify these possibilities and direct targets of therapy for patients with CRS, regardless of other underlying conditions.
Acknowledgments
Funding/Support: This study was supported by the Cystic Fibrosis Foundation (Dr Peña) and National Institutes of Health General Clinical Research Center grant GCRC 5-MO1-RR-020358-02. Confocal microscopy imaging was supported by core grant 1P30HD40677 to the Children’s Mental Retardation and Developmental Disabilities Research Center.
Footnotes
Author Contributions: Dr Wu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Wu, Rose, and Peña. Acquisition of data: Wu, Amorn, Aujla, Rice, Mimms, Peters-Hall, and Peña. Analysis and interpretation of data: Wu, Amorn, Watson, Peters-Hall, Rose, and Peña. Drafting of the manuscript: Wu, Amorn, Rice, and Rose. Critical revision of the manuscript for important intellectual content: Wu, Amorn, Aujla, Watson, Peters-Hall, Rose, and Peña. Statistical analysis: Watson. Obtained funding: Rose and Peña. Administrative, technical, and material support: Wu, Amorn, Aujla, Rice, Mimms, and Peña. Study supervision: Wu, Peters-Hall, Rose, and Peña.
Financial Disclosure: None reported.
Previous Presentation: The study was presented at the meeting of the American Society of Pediatric Otolaryngology; May 1, 2010; Las Vegas, Nevada.
Additional Contributions: April Harrison-Boddie, MS, provided administrative support. Members of the Otolaryngology, Neurosurgery, and Plastic Surgery Divisions at Children’s National Medical Center provided sinus mucosal samples.
REFERENCES
- 1.Welsh MJ, Tsui L-C, Boat TF, Beaudet AL. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. 7th ed. New York: McGraw-Hill; 1995. pp. 3799–3876. [Google Scholar]
- 2.Cuyler JP. Follow-up of endoscopic sinus surgery on children with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 1992;118(5):505–506. doi: 10.1001/archotol.1992.01880050057013. [DOI] [PubMed] [Google Scholar]
- 3.Gentile VG, Isaacson G. Patterns of sinusitis in cystic fibrosis. Laryngoscope. 1996;106(8):1005–1009. doi: 10.1097/00005537-199608000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Boucher RC, Knowles MR, Yankaskas JR. Cystic fibrosis. In: Mason RJ, editor. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Philadelphia, PA: Saunders Elsevier; 2010. pp. 576–592. [Google Scholar]
- 5.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86(1):245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 6.Bedrossian CW, Greenberg SD, Singer DB, Hansen JJ, Rosenberg HS. The lung in cystic fibrosis: a quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol. 1976;7(2):195–204. doi: 10.1016/s0046-8177(76)80023-8. [DOI] [PubMed] [Google Scholar]
- 7.Sobonya RE, Taussig LM. Quantitative aspects of lung pathology in cystic fibrosis. Am Rev Respir Dis. 1986;134(2):290–295. doi: 10.1164/arrd.1986.134.2.290. [DOI] [PubMed] [Google Scholar]
- 8.Tos M, Mogensen C. Mucus production in chronic maxillary sinusitis: a quantitative histopathological study. Acta Otolaryngol. 1984;97(1–2):151–159. doi: 10.3109/00016488409130975. [DOI] [PubMed] [Google Scholar]
- 9.Peña MT, Aujla PK, Zudaire E, et al. Localization and expression of MUC5B and MUC7 mucins in pediatric sinus mucosa. Ann Otol Rhinol Laryngol. 2007;116(5):389–397. doi: 10.1177/000348940711600513. [DOI] [PubMed] [Google Scholar]
- 10.Peña MT, Aujla PK, Patel KM, Zalzal GH, Rose MC. Immunohistochemical analyses of MUC5AC mucin expression in sinus mucosa of children with sinusitis and controls. Ann Otol Rhinol Laryngol. 2005;114(12):958–965. doi: 10.1177/000348940511401212. [DOI] [PubMed] [Google Scholar]
- 11.Litt M, Khan MA, Chakrin LW, Wardell JR, Jr, Christian P. The viscoelasticity of fractionated canine tracheal mucus. Biorheology. 1974;11(2):111–117. doi: 10.3233/bir-1974-11202. [DOI] [PubMed] [Google Scholar]
- 12.Rose MC, Lynn WS, Kaufman B. Resolution of the major components of human lung mucosal gel and their capabilities for reaggregation and gel formation. Biochemistry. 1979;18(18):4030–4037. doi: 10.1021/bi00585a029. [DOI] [PubMed] [Google Scholar]
- 13.Davies JR, Herrmann A, Russell W, Svitacheva N, Wickstrom C, Carlstedt I. Respiratory tract mucins: structure and expression patterns. Novartis Found Symp. 2002;248:76–93. 277–282. [PubMed] [Google Scholar]
- 14.Sharma P, Dudus L, Nielsen PA, et al. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am J Respir Cell Mol Biol. 1998;19(1):30–37. doi: 10.1165/ajrcmb.19.1.3054. [DOI] [PubMed] [Google Scholar]
- 15.Viswanathan H, Brownlee IA, Pearson JP, Carrie S. MUC5B secretion is up-regulated in sinusitis compared with controls. Am J Rhinol. 2006;20(5):554–557. doi: 10.2500/ajr.2006.20.2935. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Antón A, Debolós C, Garrido M, et al. Mucin genes have different expression patterns in healthy and diseased upper airway mucosa. Clin Exp Allergy. 2006;36(4):448–457. doi: 10.1111/j.1365-2222.2006.02451.x. [DOI] [PubMed] [Google Scholar]
- 17.Lund VJ, Kennedy DW. Staging and Therapy Group. Quantification for staging sinusitis. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 18.Lusk RP, Stankiewicz JA. Pediatric rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3, pt 2):S53–S57. doi: 10.1016/S0194-59989770008-1. [DOI] [PubMed] [Google Scholar]
- 19.Ho SB, Roberton AM, Shekels LL, Lyftogt CT, Niehans GA, Toribara NW. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995;109(3):735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 20.Piludu M, Rayment SA, Liu B, et al. Electron microscopic immunogold localization of salivary mucins MG1 and MG2 in human submandibular and sublingual glands. J Histochem Cytochem. 2003;51(1):69–79. doi: 10.1177/002215540305100109. [DOI] [PubMed] [Google Scholar]
- 21.Baroody FM, Hughes CA, McDowell P, Hruban R, Zinreich SJ, Naclerio RM. Eosinophilia in chronic childhood sinusitis. Arch Otolaryngol Head Neck Surg. 1995;121(12):1396–1402. doi: 10.1001/archotol.1995.01890120054010. [DOI] [PubMed] [Google Scholar]
- 22.Spicer SS, Chakrin LW, Wardell JR, Jr, Kendrick W. Histochemistry of mucosubstances in the canine and human respiratory tract. Lab Invest. 1971;25(6):483–490. [PubMed] [Google Scholar]
- 23.Wright JM, Merlo CA, Reynolds JB, et al. Respiratory epithelial gene expression in patients with mild and severe cystic fibrosis lung disease. Am J Respir Cell Mol Biol. 2006;35(3):327–336. doi: 10.1165/rcmb.2005-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124(1):37–42. doi: 10.1016/j.jaci.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahoney EJ, Metson R. Palliative care for the patient with refractory chronic rhinosinusitis. Otolaryngol Clin North Am. 2009;42(1):39–47. vii–viii. doi: 10.1016/j.otc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Antón A, Roca-Ferrer J, Mullol J. Mucin gene expression in rhinitis syndromes. Curr Allergy Asthma Rep. 2006;6(3):189–197. doi: 10.1007/s11882-006-0034-3. [DOI] [PubMed] [Google Scholar]
- 27.Ali MS, Pearson JP. Upper airway mucin gene expression: a review. Laryngoscope. 2007;117(5):932–938. doi: 10.1097/MLG.0b013e3180383651. [DOI] [PubMed] [Google Scholar]
- 28.Casalino-Matsuda SM, Monzon ME, Day AJ, Forteza RM. Hyaluronan fragments/CD44mediate oxidative stress-induced MUC5B up-regulation in airway epithelium. Am J Respir Cell Mol Biol. 2009;40(3):277–285. doi: 10.1165/rcmb.2008-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]