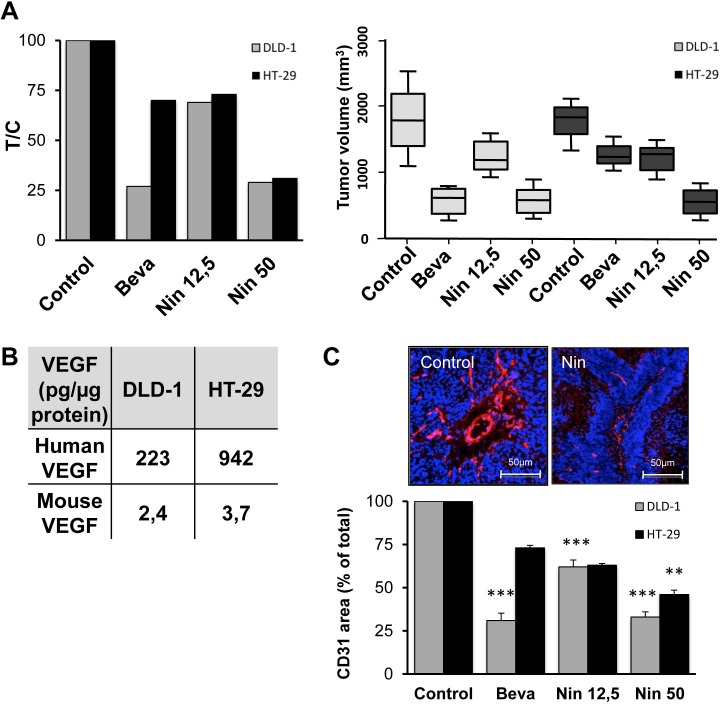
Figure 1. Influence of bevacizumab and nintedanib on tumor growth and angiogenesis in CRC xenografts.
(A) Nude mice with DLD-1 (grey columns) or HT-29 (black columns) human CRC xenografts were dosed with vehicle (Control), bevacizumab at 5 mg/kg i.p. every 3 days (Beva), nintedanib at 12,5 mg/kg p.o. once daily (Nin 12,5) or nintedanib at 50 mg/kg p.o. once daily (Nin 50) for 4 weeks. Each treatment group corresponded to at least seven animals. Left, T/C values were determined as follows: average tumor volume of treated animals/average tumor volume of the vehicle controls x100. Right, box and whisker plot of the tumor volumes of DLD-1 (light grey boxes) or HT-29 (dark grey boxes) xenografts after 4 weeks treatment with bevacizumab or nintedanib. Lines, medians; boxes, 25th to 75th percentile interquartile ranges; whiskers, the highest and lowest values for a given treatment. (B) Total protein was extracted from DLD-1 and HT-29 xenografts (pool of 3 tumors per xenograft model) and the amounts of human (tumor-derived) and murine (stroma-derived) VEGF were determined by ELISA. (C) Top, representative images of xenografts from animals treated with vehicle or with nintedanib at 50 mg/kg p.o. once daily. CD31-positive blood vessels are outlined in red whereas the nuclei appear in blue. Bottom, quantitative image analysis of the CD31 signal for DLD-1 (grey columns) and HT-29 tumors (black columns). The data show the CD31-positive area, as % of total, and represent the average of at least 6 fields/tumor for at least 3 different tumors. The statistical analysis of experimental data was performed using a Student's paired t-test comparing the treatment group with the vehicle control. Bars, SD; * p < 0,05; ** p < 0,01; *** p < 0,001.