Abstract
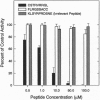
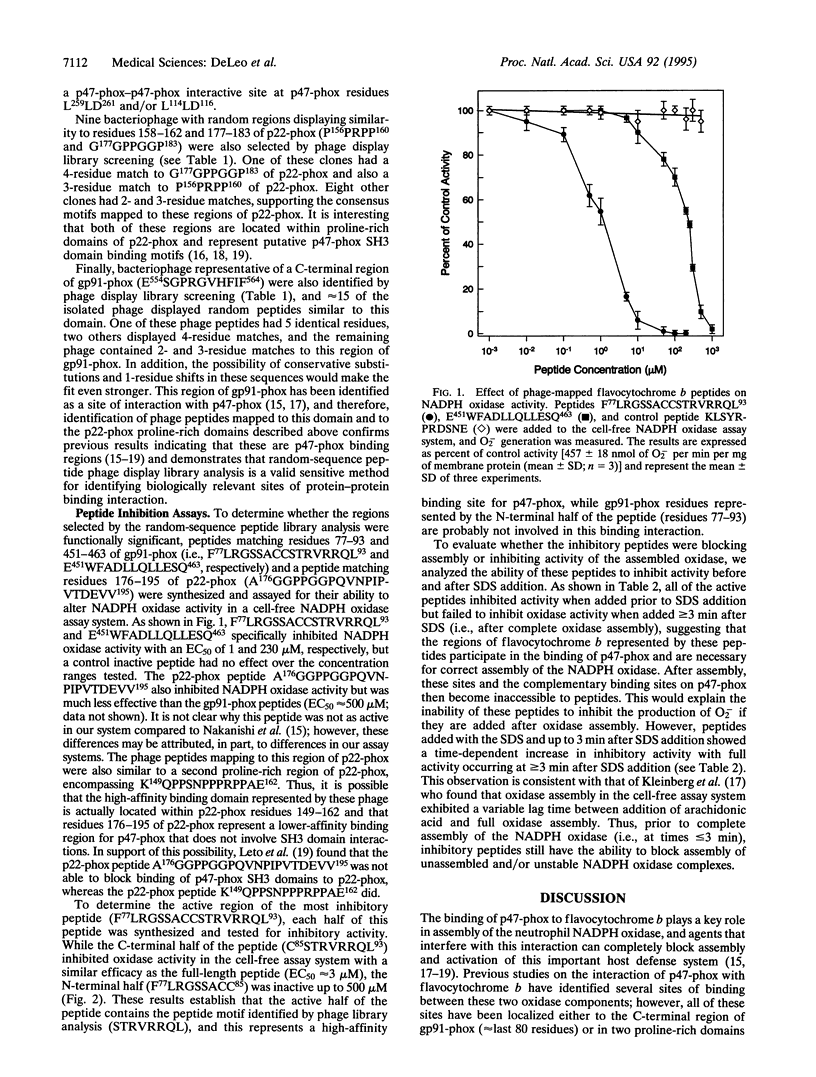
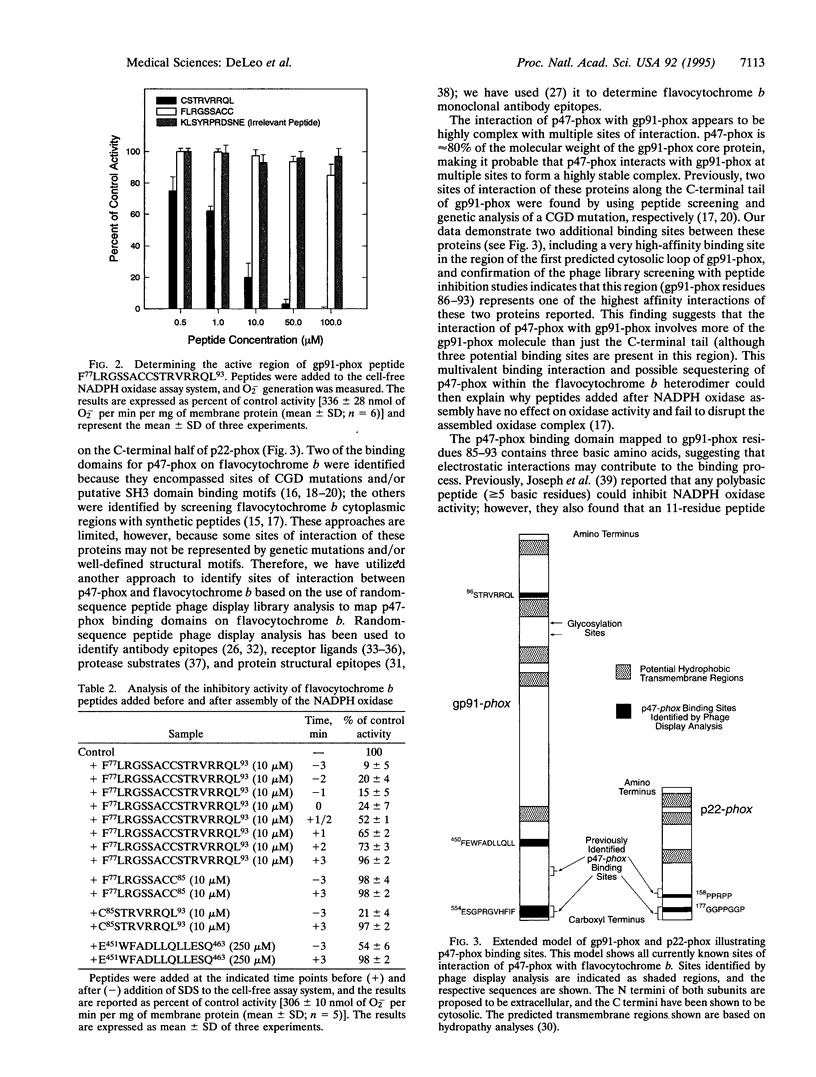
During assembly of the phagocyte NADPH oxidase, cytosolic p47-phox translocates to the plasma membrane and binds to flavocytochrome b, and binding domains for p47-phox have been identified on the C-terminal tails of both flavocytochrome b subunits. In the present report, we further examine the interaction of these two oxidase components by using random-sequence peptide phage display library analysis. Screening p47-phox with the peptide libraries identified five potential sites of interaction with flavocytochrome b, including three previously reported regions of interaction and two additional regions of interaction of p47-phox with gp91-phox and p22-phox. The additional sites were mapped to a domain on the first predicted cytosolic loop of gp91-phox encompassing residues S86TRVRRQL93 and to a domain near the cytosolic C-terminal tail of gp91-phox encompassing residues F450EWFADLL457. The mapping also confirmed a previously reported binding domain on gp91-phox (E554SGPRGVHFIF564) and putative Src homology 3 domain binding sites on p22-phox (P156PRPP160 and G177GPPGGP183). To demonstrate that the additional regions identified were biologically significant, peptides mimicking the gp91-phox sequences F77LRGSSACCSTRVRRQL93 and E451WFADLLQLLESQ463 were synthesized and assayed for their ability to inhibit NADPH oxidase activity. These peptides had EC50 values of 1 microM and 230 microM, respectively, and inhibited activation when added prior to assembly but did not affect activity of the preassembled oxidase. Our data demonstrate the usefulness of phage display library analysis for the identification of biologically relevant sites of protein-protein interaction and show that the binding of p47-phox to flavocytochrome b involves multiple binding sites along the C-terminal tails of both gp91- and p22-phox and other regions of gp91-phox nearer to the N terminus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991 Oct 17;353(6345):668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Boulay F., Badwey J. A., Curnutte J. T. Activation of neutrophil leukocytes: chemoattractant receptors and respiratory burst. FASEB J. 1993 Aug;7(11):1004–1010. doi: 10.1096/fasebj.7.11.8396540. [DOI] [PubMed] [Google Scholar]
- Chanock S. J., el Benna J., Smith R. M., Babior B. M. The respiratory burst oxidase. J Biol Chem. 1994 Oct 7;269(40):24519–24522. [PubMed] [Google Scholar]
- Cheadle C., Ivashchenko Y., South V., Searfoss G. H., French S., Howk R., Ricca G. A., Jaye M. Identification of a Src SH3 domain binding motif by screening a random phage display library. J Biol Chem. 1994 Sep 30;269(39):24034–24039. [PubMed] [Google Scholar]
- Clark R. A. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990 Jun;161(6):1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Volpp B. D., Leidal K. G., Nauseef W. M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J Clin Invest. 1990 Mar;85(3):714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwirla S. E., Peters E. A., Barrett R. W., Dower W. J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J. J., Panganiban L. C., Devlin P. E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990 Jul 27;249(4967):404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- Dinauer M. C. The respiratory burst oxidase and the molecular genetics of chronic granulomatous disease. Crit Rev Clin Lab Sci. 1993;30(4):329–369. doi: 10.3109/10408369309082591. [DOI] [PubMed] [Google Scholar]
- Finan P., Shimizu Y., Gout I., Hsuan J., Truong O., Butcher C., Bennett P., Waterfield M. D., Kellie S. An SH3 domain and proline-rich sequence mediate an interaction between two components of the phagocyte NADPH oxidase complex. J Biol Chem. 1994 May 13;269(19):13752–13755. [PubMed] [Google Scholar]
- Fujita I., Takeshige K., Minakami S. Characterization of the NADPH-dependent superoxide production activated by sodium dodecyl sulfate in a cell-free system of pig neutrophils. Biochim Biophys Acta. 1987 Oct 22;931(1):41–48. doi: 10.1016/0167-4889(87)90048-6. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Curnutte J. T., Nauseef W. M., Volpp B. D., Pearson D. W., Rosen H., Clark R. A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J Clin Invest. 1991 Jan;87(1):352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess R. H., Mack A. J., Walton H., Reilly T. M. Identification of a structural epitope by using a peptide library displayed on filamentous bacteriophage. J Immunol. 1994 Jul 15;153(2):724–729. [PubMed] [Google Scholar]
- Joseph G., Gorzalczany Y., Koshkin V., Pick E. Inhibition of NADPH oxidase activation by synthetic peptides mapping within the carboxyl-terminal domain of small GTP-binding proteins. Lack of amino acid sequence specificity and importance of polybasic motif. J Biol Chem. 1994 Nov 18;269(46):29024–29031. [PubMed] [Google Scholar]
- Kleinberg M. E., Malech H. L., Rotrosen D. The phagocyte 47-kilodalton cytosolic oxidase protein is an early reactant in activation of the respiratory burst. J Biol Chem. 1990 Sep 15;265(26):15577–15583. [PubMed] [Google Scholar]
- Knaus U. G., Heyworth P. G., Evans T., Curnutte J. T., Bokoch G. M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991 Dec 6;254(5037):1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- Koivunen E., Wang B., Ruoslahti E. Isolation of a highly specific ligand for the alpha 5 beta 1 integrin from a phage display library. J Cell Biol. 1994 Feb;124(3):373–380. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto T. L., Adams A. G., de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto T. L., Garrett M. C., Fujii H., Nunoi H. Characterization of neutrophil NADPH oxidase factors p47-phox and p67-phox from recombinant baculoviruses. J Biol Chem. 1991 Oct 15;266(29):19812–19818. [PubMed] [Google Scholar]
- Leusen J. H., Bolscher B. G., Hilarius P. M., Weening R. S., Kaulfersch W., Seger R. A., Roos D., Verhoeven A. J. 156Pro-->Gln substitution in the light chain of cytochrome b558 of the human NADPH oxidase (p22-phox) leads to defective translocation of the cytosolic proteins p47-phox and p67-phox. J Exp Med. 1994 Dec 1;180(6):2329–2334. doi: 10.1084/jem.180.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leusen J. H., de Boer M., Bolscher B. G., Hilarius P. M., Weening R. S., Ochs H. D., Roos D., Verhoeven A. J. A point mutation in gp91-phox of cytochrome b558 of the human NADPH oxidase leading to defective translocation of the cytosolic proteins p47-phox and p67-phox. J Clin Invest. 1994 May;93(5):2120–2126. doi: 10.1172/JCI117207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. J., Wells J. A. Substrate phage: selection of protease substrates by monovalent phage display. Science. 1993 May 21;260(5111):1113–1117. doi: 10.1126/science.8493554. [DOI] [PubMed] [Google Scholar]
- Nakanishi A., Imajoh-Ohmi S., Fujinawa T., Kikuchi H., Kanegasaki S. Direct evidence for interaction between COOH-terminal regions of cytochrome b558 subunits and cytosolic 47-kDa protein during activation of an O(2-)-generating system in neutrophils. J Biol Chem. 1992 Sep 25;267(27):19072–19074. [PubMed] [Google Scholar]
- Nauseef W. M., McCormick S., Renee J., Leidal K. G., Clark R. A. Functional domain in an arginine-rich carboxyl-terminal region of p47phox. J Biol Chem. 1993 Nov 5;268(31):23646–23651. [PubMed] [Google Scholar]
- Nunoi H., Rotrosen D., Gallin J. I., Malech H. L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988 Dec 2;242(4883):1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- Parkos C. A., Allen R. A., Cochrane C. G., Jesaitis A. J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987 Sep;80(3):732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Evans T., Loetterle L. R., Jesaitis A. J., Bokoch G. M. Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J Biol Chem. 1993 Oct 5;268(28):20983–20987. [PubMed] [Google Scholar]
- Quinn M. T., Parkos C. A., Jesaitis A. J. The lateral organization of components of the membrane skeleton and superoxide generation in the plasma membrane of stimulated human neutrophils. Biochim Biophys Acta. 1989 Dec 11;987(1):83–94. doi: 10.1016/0005-2736(89)90458-6. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Yeung C. L., Leto T. L., Malech H. L., Kwong C. H. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992 Jun 5;256(5062):1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- Scott J. K., Smith G. P. Searching for peptide ligands with an epitope library. Science. 1990 Jul 27;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Segal A. W., West I., Wientjes F., Nugent J. H., Chavan A. J., Haley B., Garcia R. C., Rosen H., Scrace G. Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochem J. 1992 Jun 15;284(Pt 3):781–788. doi: 10.1042/bj2840781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P., Scott J. K. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 1993;217:228–257. doi: 10.1016/0076-6879(93)17065-d. [DOI] [PubMed] [Google Scholar]
- Sparks A. B., Quilliam L. A., Thorn J. M., Der C. J., Kay B. K. Identification and characterization of Src SH3 ligands from phage-displayed random peptide libraries. J Biol Chem. 1994 Sep 30;269(39):23853–23856. [PubMed] [Google Scholar]
- Sumimoto H., Kage Y., Nunoi H., Sasaki H., Nose T., Fukumaki Y., Ohno M., Minakami S., Takeshige K. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig R., Gilman A. G. Mammalian membrane-bound adenylyl cyclases. J Biol Chem. 1995 Jan 6;270(1):1–4. doi: 10.1074/jbc.270.1.1. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Clark R. A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988 Dec 2;242(4883):1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- Yayon A., Aviezer D., Safran M., Gross J. L., Heldman Y., Cabilly S., Givol D., Katchalski-Katzir E. Isolation of peptides that inhibit binding of basic fibroblast growth factor to its receptor from a random phage-epitope library. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10643–10647. doi: 10.1073/pnas.90.22.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Takeshige K., Nunoi H., Minakami S. Characterization of the GTP-dependent activation of the superoxide-producing NADPH oxidase in a cell-free system of pig neutrophils. Biochim Biophys Acta. 1993 Jul 28;1178(1):73–80. doi: 10.1016/0167-4889(93)90111-2. [DOI] [PubMed] [Google Scholar]