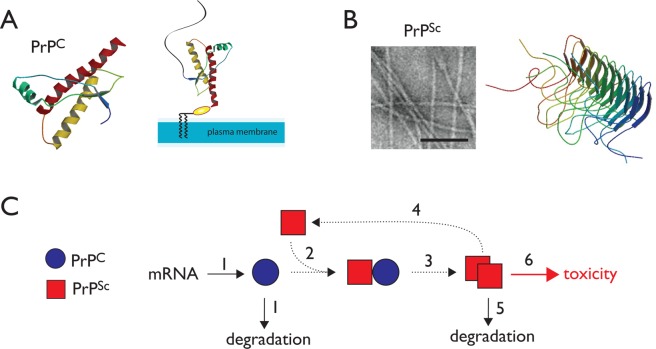
Figure 1.
Mechanism of prion propagation and potential points of therapeutic intervention. (A) The NMR structure of the folded domain of human PrPC is shown. (B) PrPSc forms fibrillar aggregates as shown in the electron micrograph (bar indicates 1000 Å). The β solenoid structure of the fungal Het-S prion provides one possible model of the pathogenic fold. (C) A simplified model for the propagation of PrPSc suggests several potential sites for therapeutic intervention.