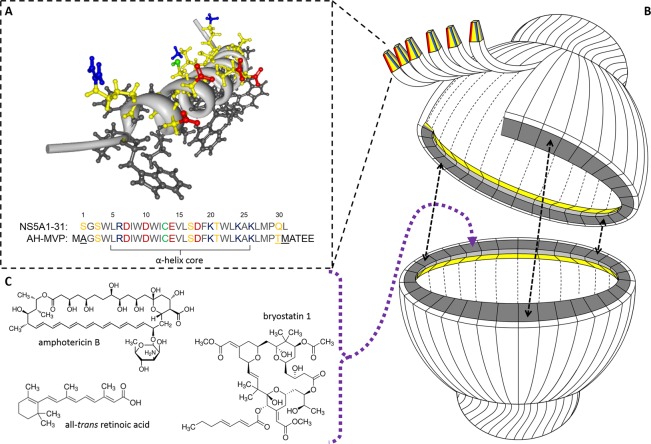
Figure 1.
(A) NMR-resolved three-dimensional structure of the hepatitis C NS5A1-31 amphipathic α-helix domain highlighting its asymmetric charge distribution along the polar face (PDB 1R7E). Hydrophobic residues are in black, while polar residues are in yellow with acidic (Glu/Asp) and basic (Arg/Lys) functional groups in red and blue, respectively. The cysteine residue SH is in green. (B) Simplified cartoon cutaway schematic of an AH vault depicting the attachment of NS5A1-31 amphipathic α-helix to MVP with subsequent vault self-assembly and self-association of adjacent NS5A1-31 amphipathic α-helices to form an internalized lipophilic ring for sequestering small therapeutic compounds. (C) Three sample therapeutic compounds that become reversibly associated within AH vaults.