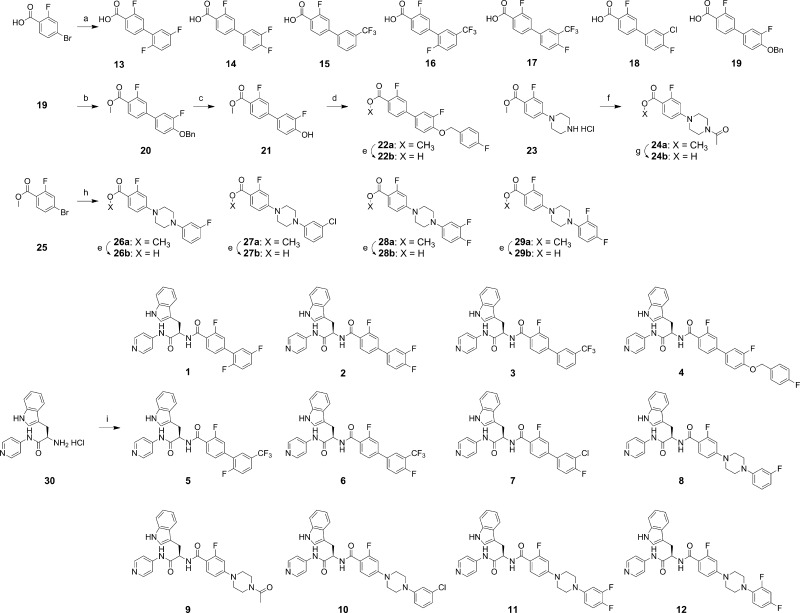
Scheme 1. Syntheses of Compounds 1–12.
Reagents and conditions: (a) arylboronic acid, 5 mol % Pd2(dba)3, 10 mol % PCy3, 2M K3PO4, dioxane, 100 °C (microwave), 1 h, ca. 90%; (b) H2SO4/MeOH (1/10), 70 °C, 24 h, 91%; (c) H2 (balloon), Pd/C, MeOH–acetone, 23 °C, 24 h, 92%; (d) 4-fluorobenzyl bromide, K2CO3, acetone, 70 °C, 5 h, 95%; (e) 10% NaOH (aq), MeOH/THF (1/1), 60 °C, 3 h, ca. 95%; (f) acetic anhydride, Et3N, CH2Cl2, 0–23 °C, 1 h, 84%; (g) 10% NaOH (aq), MeOH/THF (1/1), 23 °C, 2 h, 36%; (h) 1-(aryl)piperazine, Pd(OAc)2, P(o-tolyl)3, Cs2CO3, toluene, 60 °C, 48 h, ca. 70%; (i) 13, 14, 15, 16, 17, 18, 19, 22b, 24b, 26b, 27b, 28b, or 29b (as appropriate), PyBOP, HOBt, Et3N, CH2Cl2, 23 °C, 1 h, ca. 70%.