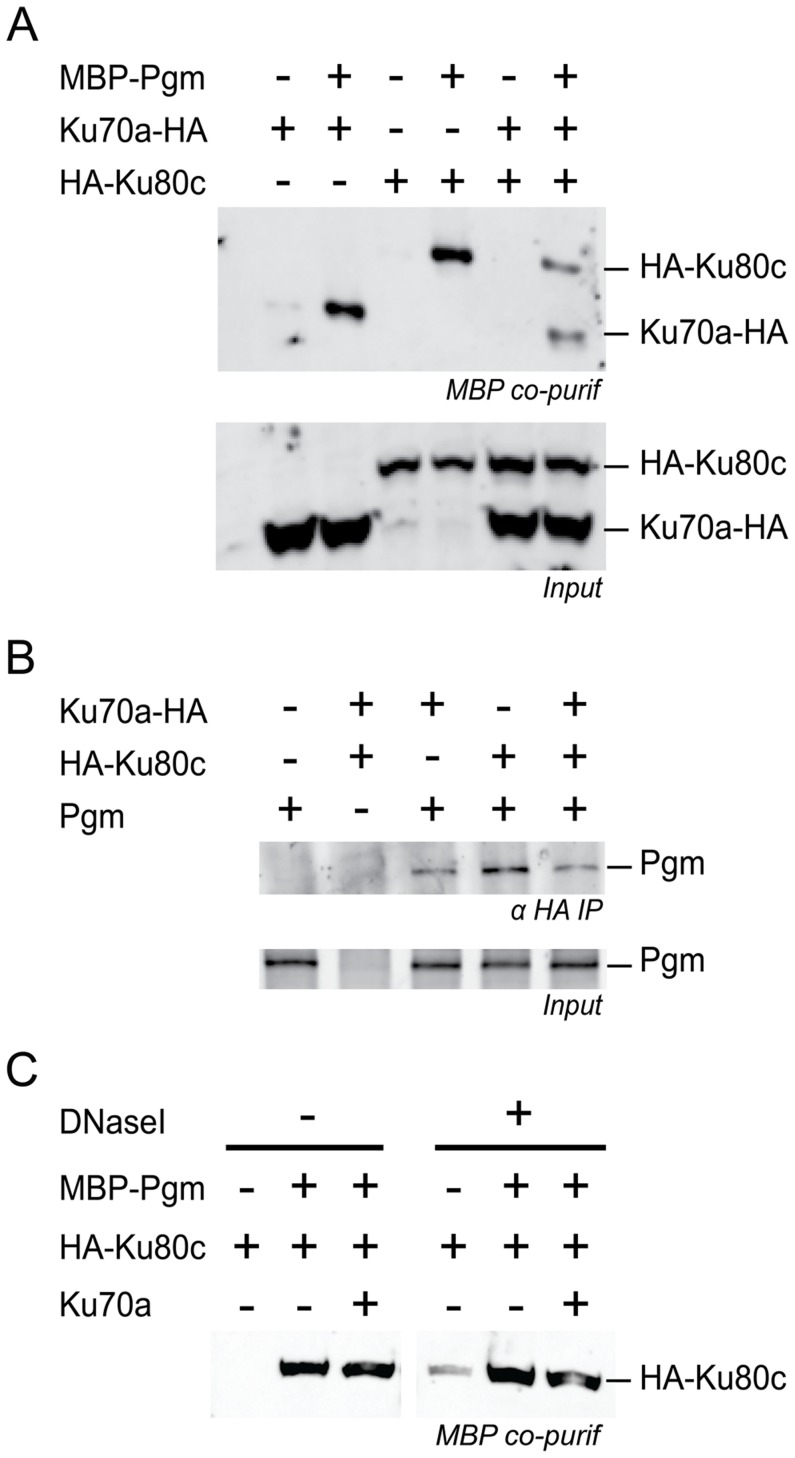
Figure 7. Physical interaction between Pgm and Ku.
(A) Co-precipitation of HA-Ku80c and Ku70a-HA with MBP-Pgm from insect cell extracts (top panel), revealed on western blots using an anti-HA antibody. (B) Co-immunoprecipitation of Pgm with HA-Ku80c and/or Ku70a-HA from insect cell extracts, revealed on western blots using an anti-Pgm antibody. In A and B, the input proteins from each extract are displayed in the bottom panel. (C) The interaction between HA-Ku80c and Pgm is resistant to DNaseI treatment. The co-precipitation experiment was performed as described in A, using MBP-Pgm, HA-Ku80c and 6His-Ku70a recombinant proteins produced from baculovirus vectors. EDTA was removed from the lysis buffer and replaced by 10 mM MgCl2. Half of the sample was treated with 40 µg/mL of Dnase I during the 2-hr incubation with amylose-coupled magnetic beads. The presence of HA-Ku80c in the purified complexes was revealed on western blots using an anti-HA antibody.