Abstract
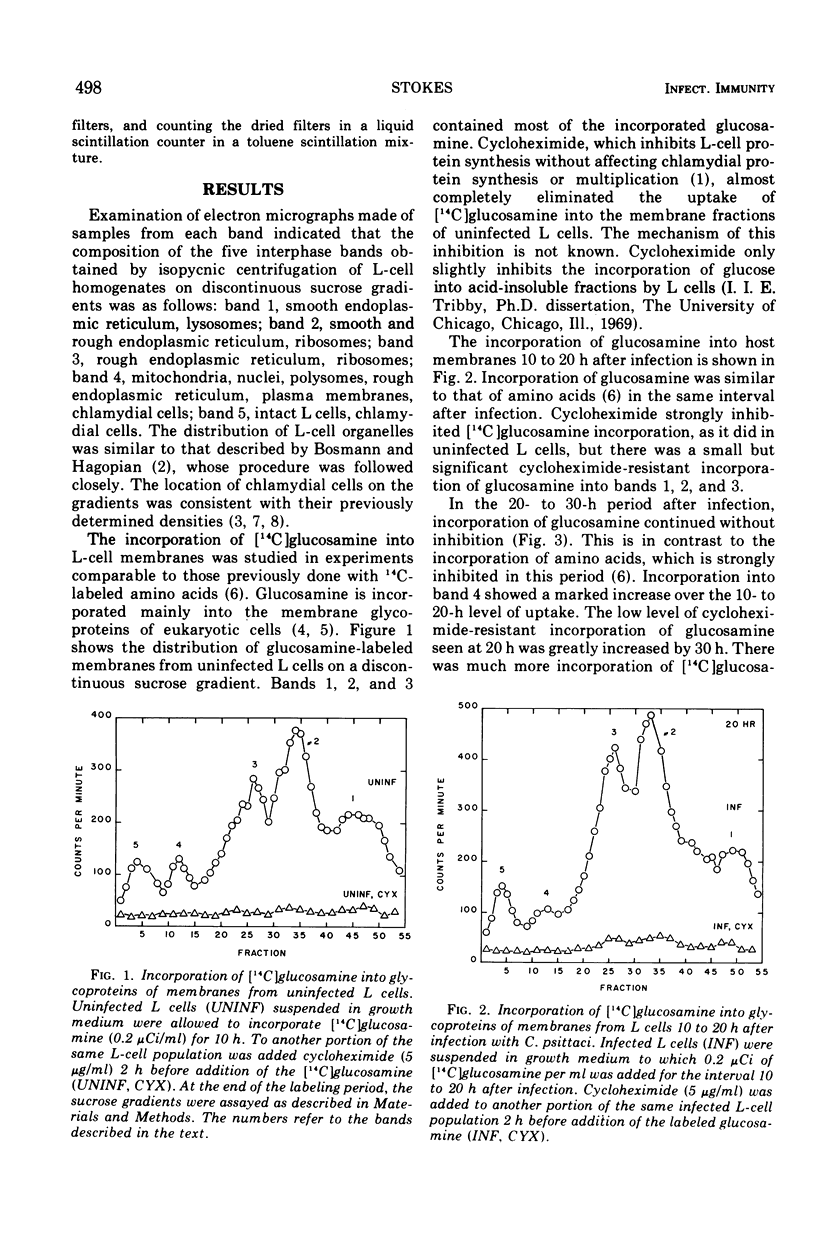
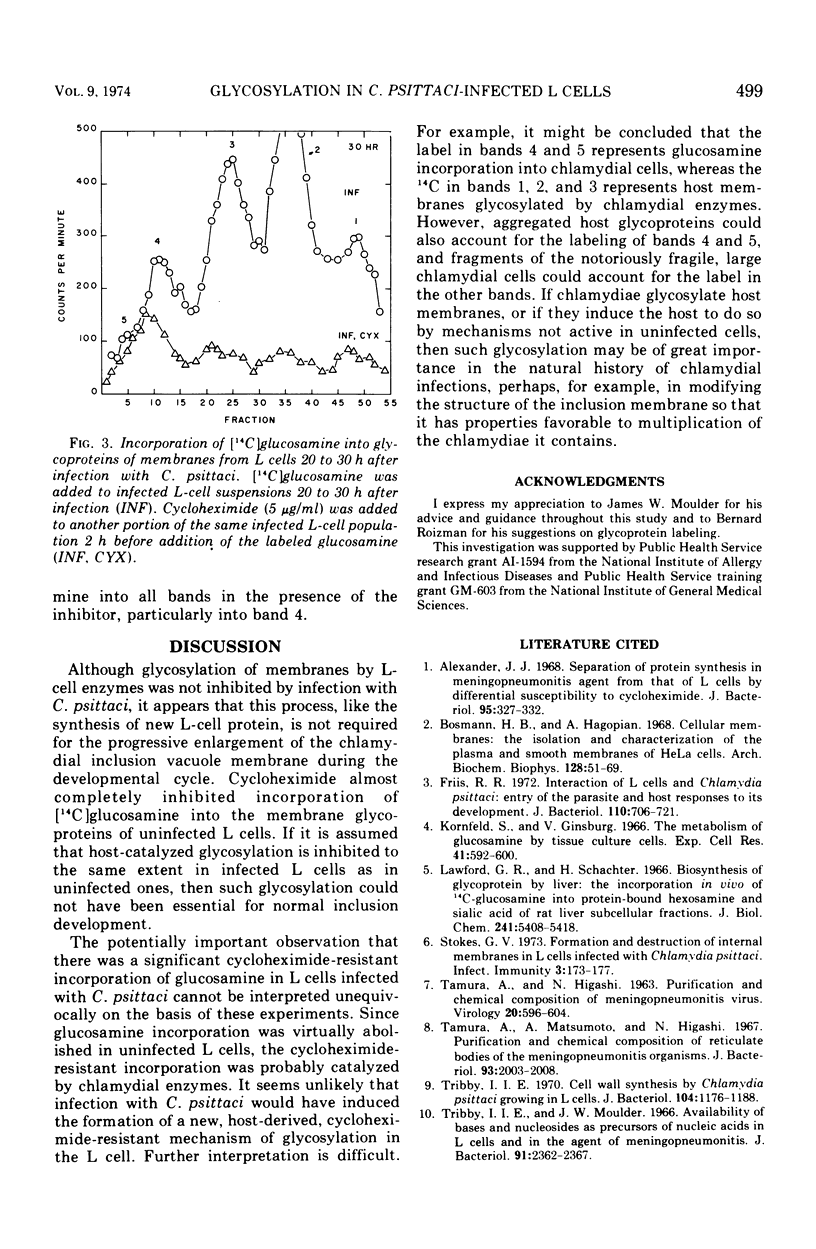
L cells (mouse fibroblasts), uninfected and infected with the meningopneumonitis strain of Chlamydia psittaci, were labeled with [14C]glucosamine, and their membranous organelles were separated by isopycnic equilibrium centrifugation of whole cell homogenates on discontinuous sucrose gradients. Glycosylation of host membranes continued throughout the infection. Cycloheximide almost completely inhibited glycosylation in uninfected L cells, but it only partially inhibited the process in infected host cells. Cycloheximide-resistant glycosylation of membrane fractions with [14C]glucosamine increased as the infection proceeded and was probably due to the action of chlamydial enzymes. Modification of host membranes by glycosylation may play a role in the natural development of chlamydial infections.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys. 1968 Oct;128(1):51–69. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Ginsburg V. The metabolism of glucosamine by tissue culture cells. Exp Cell Res. 1966 Mar;41(3):592–600. doi: 10.1016/s0014-4827(66)80109-x. [DOI] [PubMed] [Google Scholar]
- Lawford G. R., Schachter H. Biosynthesis of glycoprotein by liver. The incorporation in vivo of 14C-glucosamine into protein-bound hexosamine and sialic acid of rat liver subcellular fractions. J Biol Chem. 1966 Nov 25;241(22):5408–5418. [PubMed] [Google Scholar]
- Stokes G. V. Formation and destruction of internal membranes in L cells infected with Chlamydia psittaci. Infect Immun. 1973 Feb;7(2):173–177. doi: 10.1128/iai.7.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Higashi N. Purification and chemical composition of reticulate bodies of the meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2003–2008. doi: 10.1128/jb.93.6.2003-2008.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Availability of bases and nucleosides as precursors of nucleic acids in L cells and in the agent of meningopneumonitis. J Bacteriol. 1966 Jun;91(6):2362–2367. doi: 10.1128/jb.91.6.2362-2367.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


