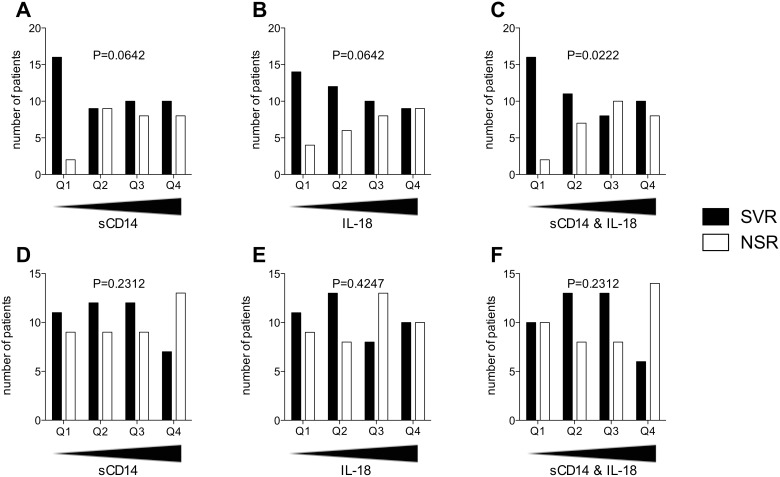
Figure 3. Associations between treatment outcome and levels of sCD14 and IL-18 in plasma: effect of Telaprevir.
Patients were ranked according to their baseline levels of sCD14, IL-18 or a combination of the two, ((x–x)/IQRx)+((y–y)/IQRy), from lowest to highest, with number of patients with a sustained virological response (SVR) or no sustained response (NSR) in each quartile assessed. (A) Number of SVR and NSR patients for increasing levels of baseline sCD14 in patients receiving dual therapy (n = 72). (B) Number of SVR and NSR patients for increasing levels of baseline IL-18 in patients receiving dual therapy (n = 72). (C) Number of SVR and NSR patients for increasing levels of the combination of the two analytes in patients receiving dual therapy (n = 72). (D) Number of SVR and NSR patients for increasing levels of baseline sCD14 in patients receiving triple therapy (n = 82). (E) Number of SVR and NSR patients for increasing levels of baseline IL-18 in patients receiving triple therapy (n = 82). (F) Number of SVR and NSR patients for increasing levels of the combination of the two analytes in patients receiving triple therapy (n = 82). P values shown represent Chi-squared test for trend.