Fig. 1.
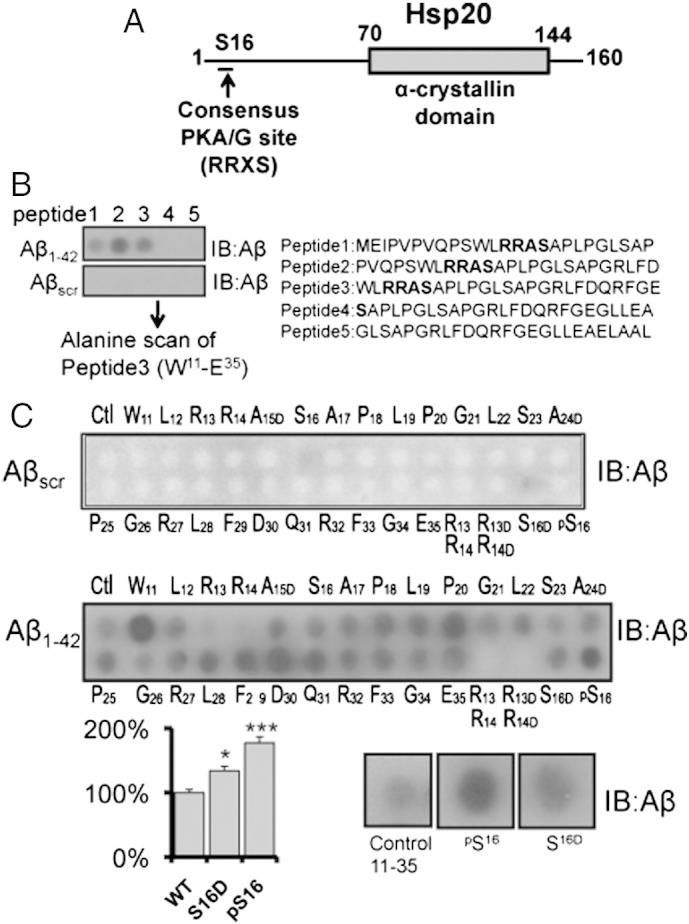
Mapping the interaction between Hsp20 and Aβ1–42.
Peptide array was used to map the domains responsible for Hsp20/Aβ1–42 interaction. (A) Diagram of domain structure of Hsp20 highlighting the PKA/PKG site located in the N-terminal domain and the conserved α-crystallin domain located between residues 70 and 144. (B) Peptide array libraries of Hsp20 25mers were probed with either Aβ1–42 or Aβscr. (C) Alanine scanning arrays of peptide 3 (W11–E35) were probed with either Aβ1–42 (middle panel) or Aβscr (upper panel) to determine the Hsp20 amino acids that are essential for Aβ1–42 binding. The association of Aβ1–42 with substitution arrays in which serine 16 was replaced by a phospho-serine or phospho-mimetic substitution (serine changed to aspartic acid) was also evaluated (lower panel). * = p < 0.05, ** = p < 0.01 using Student-t-test (n = 4).
