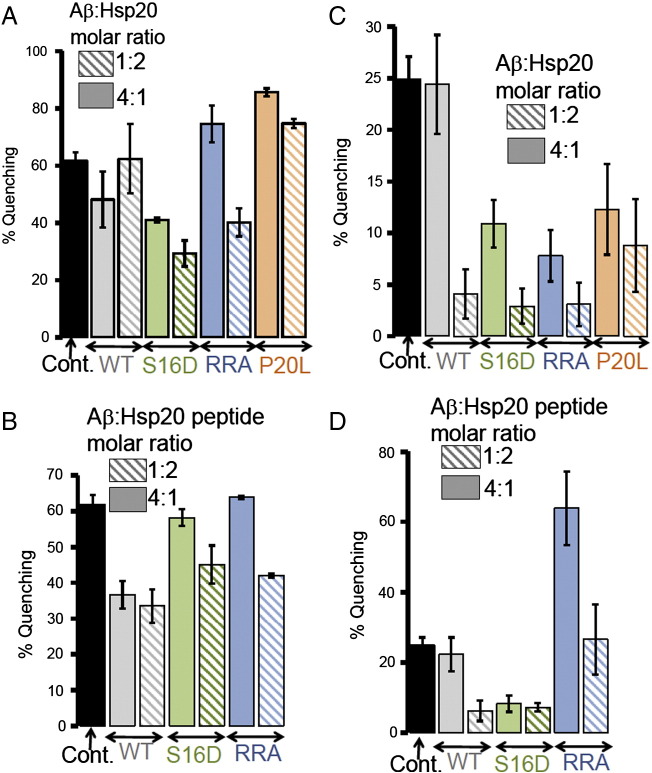
Fig. 6.
Evaluation of morphology-specific inhibition of Aβ1–42 aggregation by Hsp20 using a novel fluorescence self-quenching assay.
The interaction between Hsp20 variants and Aβ1–42 labelled at the N-terminus with HiLyte Fluor 555 (Aβ555) was monitored using fluorescence self-quenching under globular (A) and fibrillar (C) growing conditions. The interaction between Hsp20 N-terminal 25mers and Aβ1–42 labelled at the N-terminus with HiLyte Fluor 555 (Aβ555) was monitored using fluorescence self-quenching under globular (B) and fibrillar (D) growing conditions. WT = wild type Hsp20, S16D = a phosphomimetic HSP20, RRA = a construct that is defective in binding Aβ1–42, and P20L = a polymorph (a naturally occurring mutant that is known to reduce the capacity of Hsp20 to be phosphorylated at serine 16).