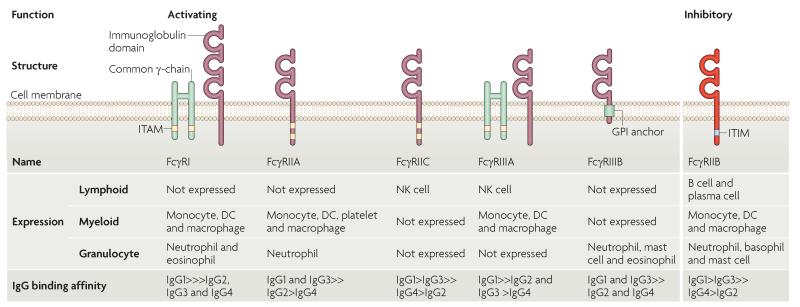
Figure 1. Structure, cellular distribution and IgG isotype-binding affinity of human activating and inhibitory FcγRs.
Human Fc receptors for IgG (FcγRs) differ in function, affinity for the Fc fragment of antibody and in cellular distribution. There are five activating FcγRs: the high-affinity receptor FcγRI, which can bind monomeric IgG, and four low-affinity receptors (FcγRIIA, FcγRIIC, FcγRIIIA and FcγRIIIB), which bind only immune-complexed IgG. Cross-linking of activating FcγRs by immune complexes results in the phosphorylation of immunoreceptor tyrosine-based activating motifs (ITAMs) that are present either in the cytoplasmic domain of the receptor (FcγRIIA and FcγRIIC), or in the associated FcR common γ-chain (FcγRI and FcγRIIIA), resulting in an activating signalling cascade. FcγRIIIB is a glycosylphosphatidylinositol (GPI)-linked receptor that has no cytoplasmic domain. FcγRIIB is the only inhibitory FcγR. It is a low affinity receptor that binds immune-complexed IgG and contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain. FcγRIIB cross-linking by immune complexes results in ITIM phosphorylation and inhibition of the activating signalling cascade. FcγRs differ in their cellular expression; myeloid cells express FcγRI, FcγRIIA and FcγRIIIA, whereas granulocytes express FcγRI, FcγRIIA and FcγRIIIB. In such cells, immune complex-mediated activation of these receptors is negatively regulated by FcγRIIB. FcγRIIB is the only FcγR expressed by B cells and negatively regulates B cell receptor activation by immune-complexed antigen. FcγRs bind different IgG subtypes with differing affinity. For example, in the case of FcγRIIB, binding affinity is highest for IgG1, followed by IgG3, which in turn has higher affinity than IgG4 followed by IgG2. The ratio of binding of an IgG subtype to activating FcγRs and inhibitory FcγRIIB is known as the A/I ratio, and it determines the activation threshold of the cell. DC, dendritic cell; NK, natural killer.