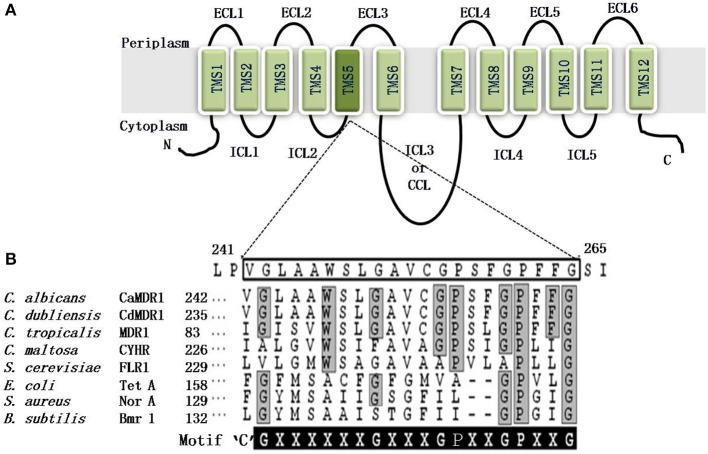
Figure 5.
(A) Predicted topology of the CaMdr1p with 12 transmembrane segments. The amino and carboxyl terminals of protein are indicated. Six extracellular loops (ECL1-6) and five intracellular loops (ICL1-5) are indicated. Two homologous halves are connected by a central cytoplasmic loop (CCL) or ICL3. The TMS5 is colored dark and magnified to show the amino acid residues of TMS5. (B) Alignment of the protein sequences of the C. albicans antiporter CaMdr1p TMS5 with the other fungal and bacterial drug antiporters, showing the presence of the unique and conserved antiporter motif or motif C. The amino acid sequence of TMS5 of CaMdr1p is boxed. The sequence of the antiporter motif is written for comparison, where X can be any amino acid. Residues conserved in all the MFS transporters that are part of the motif are highlighted in gray, whereas residues conserved only in fungal MFS that were found critical for the activity are highlighted in black.