Abstract
Purpose
The purpose of this study was to assess and compare the clinical and radiographic outcomes of guided tissue regeneration therapy for human periodontal intrabony defects using two different collagen membranes: a porous nonchemical cross-linking collagen membrane (NC) and a bilayer collagen membrane (BC).
Methods
Thirty subjects were randomly assigned and divided into the following 3 groups: a test group (NC+BM), in which a NC was used with xenograft bone mineral (BM), a positive control group (BC+BM), in which a BC was used with xenograft BM, and a negative control group (BM), in which only xenograft BM was used. The following clinical measurements were taken at baseline and 3 months after surgery: plaque index, gingival index, probing pocket depth, gingival recession, and clinical attachment level. Radiographic analysis was performed at baseline, 1 week and 3 months after surgery.
Results
Membrane exposure was not observed in any cases. Significant probing depth reduction, attachment-level gain and bone fill were observed for both test and control groups compared to baseline at 3 months after surgery (P<0.05). However, there were no statistically significant differences in clinical improvement and radiographic bone fill between treatment protocols (P>0.05).
Conclusions
Within the limitations of this study, the results suggest that both NC and BC were comparable in terms of clinical and radiographic outcomes for the treatment of periodontal intrabony defects in human subjects.
Graphical Abstract
Keywords: Chronic periodontitis, Collagen, Guided tissue regeneration
INTRODUCTION
The ultimate goal of periodontal therapy is the regeneration of the lost periodontium affected by periodontitis. However, conventional periodontal therapies usually result in tissue repair by the long junctional epithelium rather than true regeneration, that is, the formation of new cementum, new periodontal ligament and new bone [1,2]. Melcher [3] suggested that periodontal ligament cells have the capacity to regenerate the periodontal attachment [4,5]. Associated animal and human studies have established that the occlusion of cells originating from epithelium and gingival connective tissue using tissue barriers is very important in achieving periodontal regeneration [6,7].
Membranes used for the guided tissue regeneration (GTR) procedure should meet the following requirements [8]: biocompatibility, cell occlusion, integration by the host tissues, clinical manageability, and the space-making function. Nonresorbable membranes like expanded polytetrafluorethylene were quite successful in GTR therapy [9]. However, nonresorbable membranes have drawbacks, such as frequent membrane exposure and the necessity of a second surgery for membrane removal. Therefore, several resorbable membranes have been developed to overcome these drawbacks and are now widely used for guided tissue or bone regeneration [10,11].
Collagen membranes are selected frequently from the various available resorbable membranes because they have many advantageous properties including a low immune response, low toxicity, the ability to promote cellular growth and attachment, homeostasis, and the ability of collagen solutions to reconstitute into the microfibrillar structure found in natural tissues [12,13]. Nevertheless, collagen membranes are absorbed too quickly to maintain structural integrity during bone/tissue regeneration. Various physical and chemical cross-linking techniques have been applied to control the rate of collagen biodegradation, such as ultraviolet light, glutaraldehyde, and enzymatic activity. [14]. One such technique, glucose-mediated cross-linking, is a method that uses natural processes occurring in the body, and therefore may be more biocompatible than other methods [15,16]. Lee et al. [17] reported that a porous nonchemical (glucose) cross-linking collagen membrane was able to enhance bone regeneration in an animal study. However, no randomized clinical trial study was carried out to investigate the efficacy of nonchemical cross-linking collagen membranes in treating intrabony periodontal defects. A bioresorbable bilayer collagen membrane (BC) has demonstrated its potential to promote periodontal regeneration in several studies and clinical trials [18,19].
Therefore, the purpose of this randomized clinical trial was to assess and compare the clinical and radiographic outcomes of GTR therapy to treat human periodontal intrabony defects with two different collagen membranes: a nonchemical cross-linking collagen membrane and a frequently used type of BC, in combination with bovine bone mineral (BM).
MATERIALS AND METHODS
Subjects
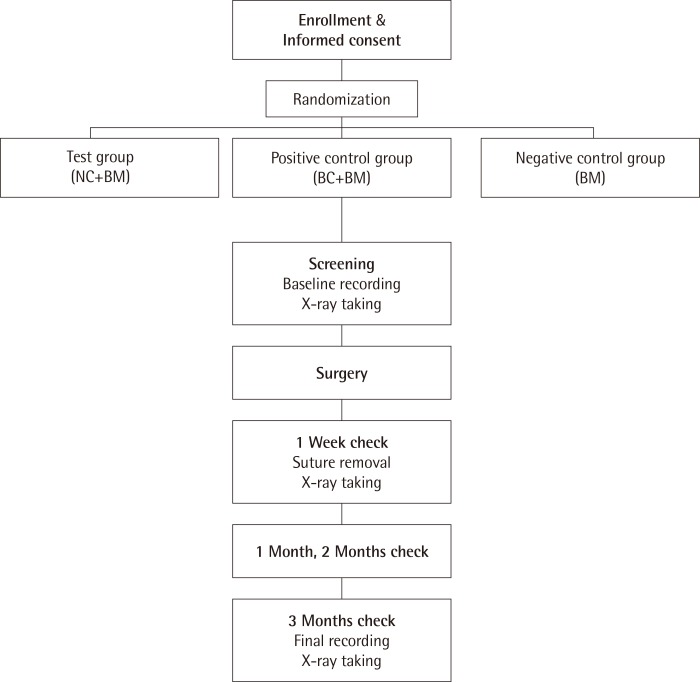
Thirty periodontitis patients who visited the Department of Periodontology, Wonkwang University Daejeon Dental Hospital, Wonkwang University School of Dentistry from October 2012 to October 2013 were enrolled in this study. Each patient was given a detailed description of the procedure and was required to sign an informed consent from prior to participation. The study protocol was approved by the Institutional Review Board of Wonkwang University Dental Hospital (IRB No. W-1202/004-005). The inclusion criteria were: (1) age between 18 and 65 and approval for surgical treatment, (2) the presence of a bone defect of more than 4 mm and probing depth over 6 mm, and (3) agreement to participate in the trial and presence of a signed consent form. The exclusion criteria were: (1) the presence of an acute abscess, (2) pregnancy or breastfeeding, (3) the presence of an orthodontic appliance, and (4) ethical issues that led to the judgment that it would be inappropriate for a given patient to participate in the clinical trial. There was a total of 30 patients, who were randomized among the three groups. To ensure randomization, we used a computer-generated randomized allocation table. There were 10 patients in the positive control group (BC+BM), which was treated with a BC (Bio-Gide, Geistilich, Wolhusen, Swiss) and a xenograft (OCS-B, NIBEC, Jincheon, Korea), and 10 patients in the test group (NC+BM), which was treated with a porous nonchemical cross-linking collagen membrane (NC) (GuidOss, NIBEC) and a xenograft (OCS-B, NIBEC). In the negative control group (BM), the 10 patients were treated with xenograft only (OCS-B, NIBEC). The flow chart of the study is shown in Fig. 1.
Figure 1.
The flow chart of the study. NC: porous nonchemical cross-linking collagen membrane, BC: bilayer collagen membrane, BM: bone mineral.
Clinical examinations
A blinded single examiner performed all measurements. Examiner reliability was assessed by re-examining 10 randomly selected patients with an interval of 10 days between measurements. A 98% agreement within 1 mm was achieved.
The following clinical measurements were made at baseline and at 3 months after surgery: plaque index (PI), gingival index (GI) [20], probing pocket depth (PD), gingival recession (GR), and clinical attachment level (CAL). The measurements were made at 6 sites per tooth. The PD was measured from the free gingiva to the pocket base. The cemento-enamel junction (CEJ) was used as a fixed reference for the GR measurement. The CAL was defined as the sum of the depth of the pocket and the GR. All examinations were performed using a periodontal probe (PW, Hu-Friedy Manufacturing Co., Chicago, IL, USA).
Radiographic analysis
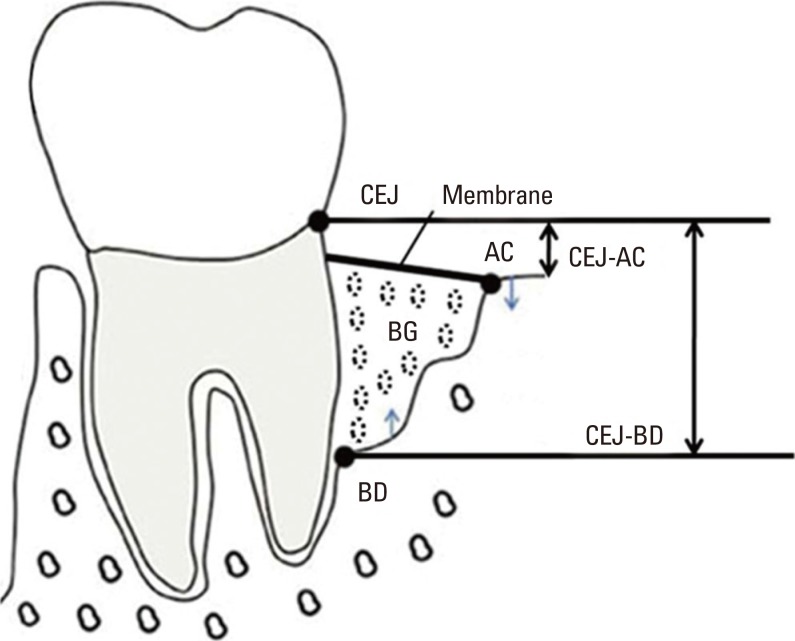
Standardized radiographs using the paralleling technique with a plastic film holder (XCP, Rinn Co., Elgin, IL, USA) were taken at baseline, 1 week, and 3 months after surgery. The variables were measured as follows: (1) the distance between the radiographic projection of the CEJ and the bottom of the defect was measured (CEJ-BD), (2) the distance between the radiographic projection of the CEJ and the alveolar crest was measured (CEJ-AC), (3) the radiographic bone fill was calculated as the difference between baseline CEJ-BD and 3 months CEJ-BD, and (4) the change in the radiographic bone level after surgery was estimated as the difference compared 1 week with 3 months for CEJ-BD and CEJ-AC, respectively (Fig. 2). The radiographs were analyzed using the PiView system (PiView STAR, Infinitt Co., Seoul, Korea).
Figure 2.
Illustration of radiographic analysis points. CEJ: cemento-enamel junction, AC: alveolar crest, BD: bottom of defect, BG: bone graft material, CEJ-AC: distance between the cement-enamel junction and the alveolar crest, CEJ-BD: distance between the cemento-enamel junction and the bottom of the defect.
Surgical procedure
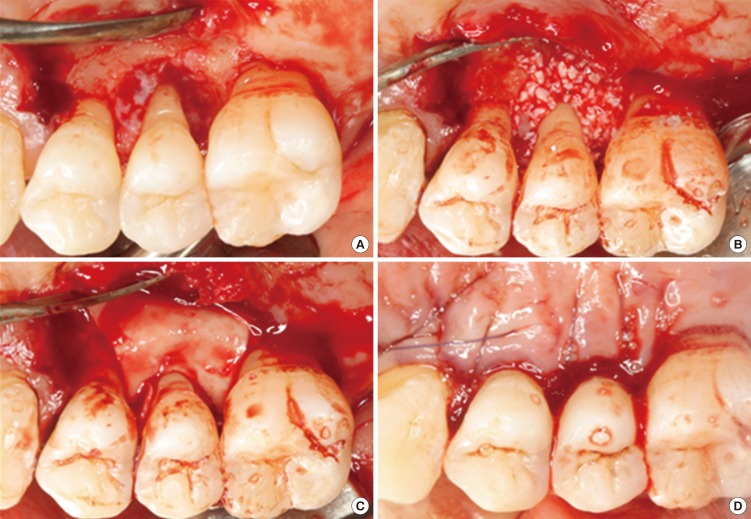
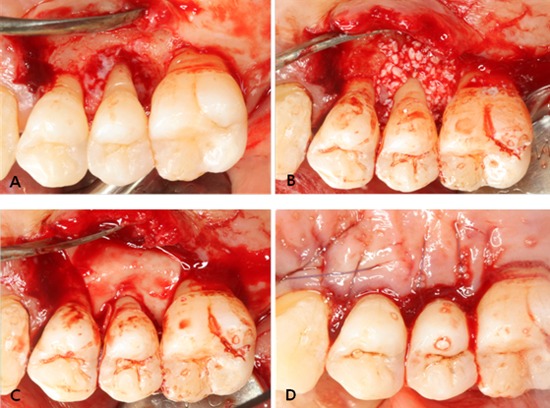
All operative procedures were performed under local anesthesia by a single surgeon. Following intracrevicular incisions, full-thickness mucoperiosteal flaps were raised vestibularly and lingually. All granulation tissue was removed from the defects and the roots were thoroughly scaled and planed using hand and ultrasonic instruments. After the surgical site was prepared, a xenograft was applied on the defect. A bioresorbable membrane was trimmed and adapted over the defect in such a manner that the entire defect and 2-3 mm of the surrounding alveolar bone were completely covered. The membrane was not sutured. The flaps were repositioned and sutured in a slightly coronally displaced position. All patients received antibiotics and analgesics for 5 days (375-mg amoxicillin and 60-mg loxoprofen). The postoperative care consisted of 0.2% chlorhexidine digluconate solution rinses twice a day for 6 weeks. Recall appointments were scheduled during the remaining observation period. Pathological tissue alterations, device exposure, or other pertinent clinical observations related to the surgery were recorded. Clinical photography was used to document the defects and progression of healing (Fig. 3).
Figure 3.
Representative intraoperative photographs of guided tissue regeneration procedure using GuidOss (NIBEC, Jincheon, Korea). (A) A deep intrabony defect on the upper second premolar was detected. The defects and root surfaces were thoroughly planed and debrided. (B) The bone mineral was applied on the intrabony defect. (C) After grafting of bone mineral, a porous nonchemical cross-linking collagen membrane (GuidOss) was trimmed and adapted over the defect. The entire defect and 2-3 mm of the surrounding alveolar bone were completely covered with a membrane. (D) The flap was sutured and completely closed without membrane exposure.
Statistical analysis
An estimate was made of the sample size from each experimental group needed to detect a radiographic bone fill difference of 1.5±1.2 mm between treatments with a significance level of 5% and a power of 80%. An estimated 20% dropout was allowed in the final sample size calculation (n=10). The data analysis was performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). The comparison of the gender proportion between groups was performed using a chi-square test. A one way analysis of variance test with 95% confidence intervals was used to identify statistically significant differences in clinical parameters and radiographic changes among the three treatment groups. Comparisons between baseline data and data from the 3-month follow-up in the test and control groups were made using the paired t-test (α=0.05).
RESULTS
Thirty subjects completed the study protocol; 20 subjects received the GTR/bone graft combination (the test and positive control groups) and 10 subjects received the bone grafting control protocol. There were no relevant differences between the test and control groups involving gender and age distribution (Table 1). Complete gingival wound closure for primary intention healing was accomplished for all defect sites. Membrane exposure was not observed in any cases. No other adverse reactions or relevant clinical findings other than GR were observed.
Table 1.
Patient characteristics.
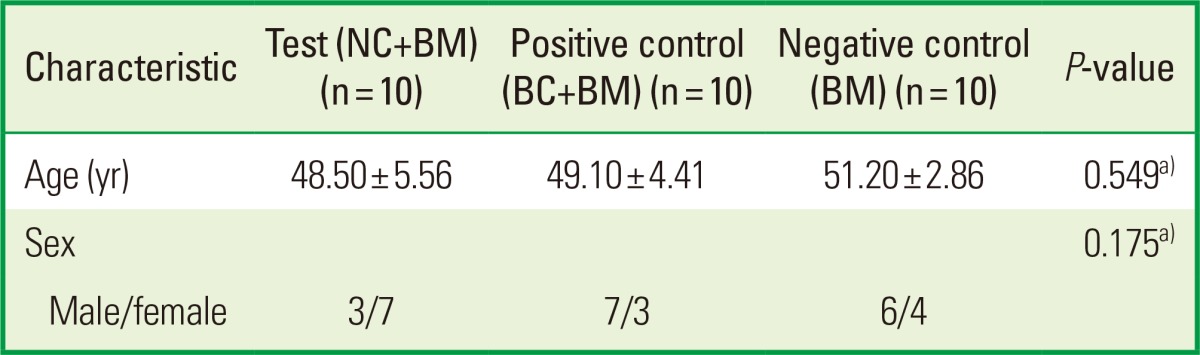
Values are presented as mean±standard deviation.
NC: porous nonchemical cross-linking collagen membrane, BC: bilayer collagen membrane, BM: bone mineral.
a)There were no statistically significant differences between the test and control groups.
The mean values of clinical parameters including PI, GI, PD, and CAL at baseline and 3 months after surgery are shown in Tables 2 and 3. There was no significant change in PI or GI compared to baseline in any treatment groups. The probing depth reduction for the NC+BM, BC+BM, and BM groups was 5.10±1.52, 3.60±1.27, and 3.60±2.12 mm, respectively. Clinical attachment level gains were 3.60±2.76, 2.50±1.72, and 2.50±3.41 mm for the NC+BM, BC+BM, and BM groups, respectively. Both the PD and CAL improved significantly compared to baseline (P<0.05) in all 3 groups at 3 months after surgery. However, there were no statistically significant differences in PD reduction or CAL gain (P>0.05) between the 3 treatment groups (Table 3). The radiographic mean bone fill was 5.83±3.68, 5.02±2.39, and 6.95±2.43 mm for the NC+BM, BC+BM, and BM groups, respectively. Although the radiographic bone fill was statistically significant compared to baseline (P<0.05) in both the test and control groups, the difference of radiographic bone fill between the treatment groups was not statistically significant (Table 4). The changes in the radiographic alveolar crest level (CEJ-AC) and the bottom level of the defect (CEJ-BD) between 1 week and 3 months after surgery are presented in Table 5. There was no statistically significant difference in bone level change between the test and control groups.
Table 2.
Clinical parameters: plaque index and gingival index.
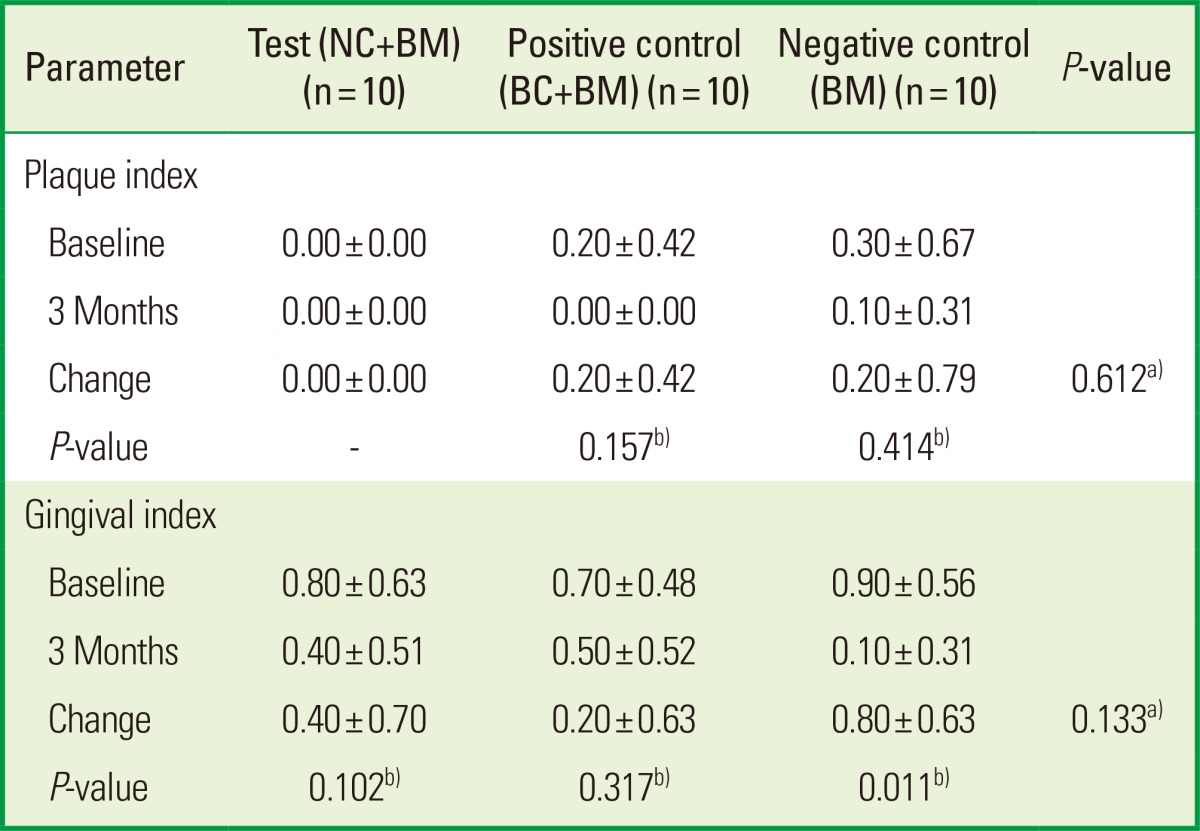
Values are presented as mean±standard deviation.
NC: porous nonchemical cross-linking collagen membrane, BC: bilayer collagen membrane, BM: bone mineral.
a)There were no statistically significant differences between the test and control groups.
b)There were no statistically significant differences compared to baseline in both test and control groups.
Table 3.
Clinical parameters: plaque index and clinical attachment level.
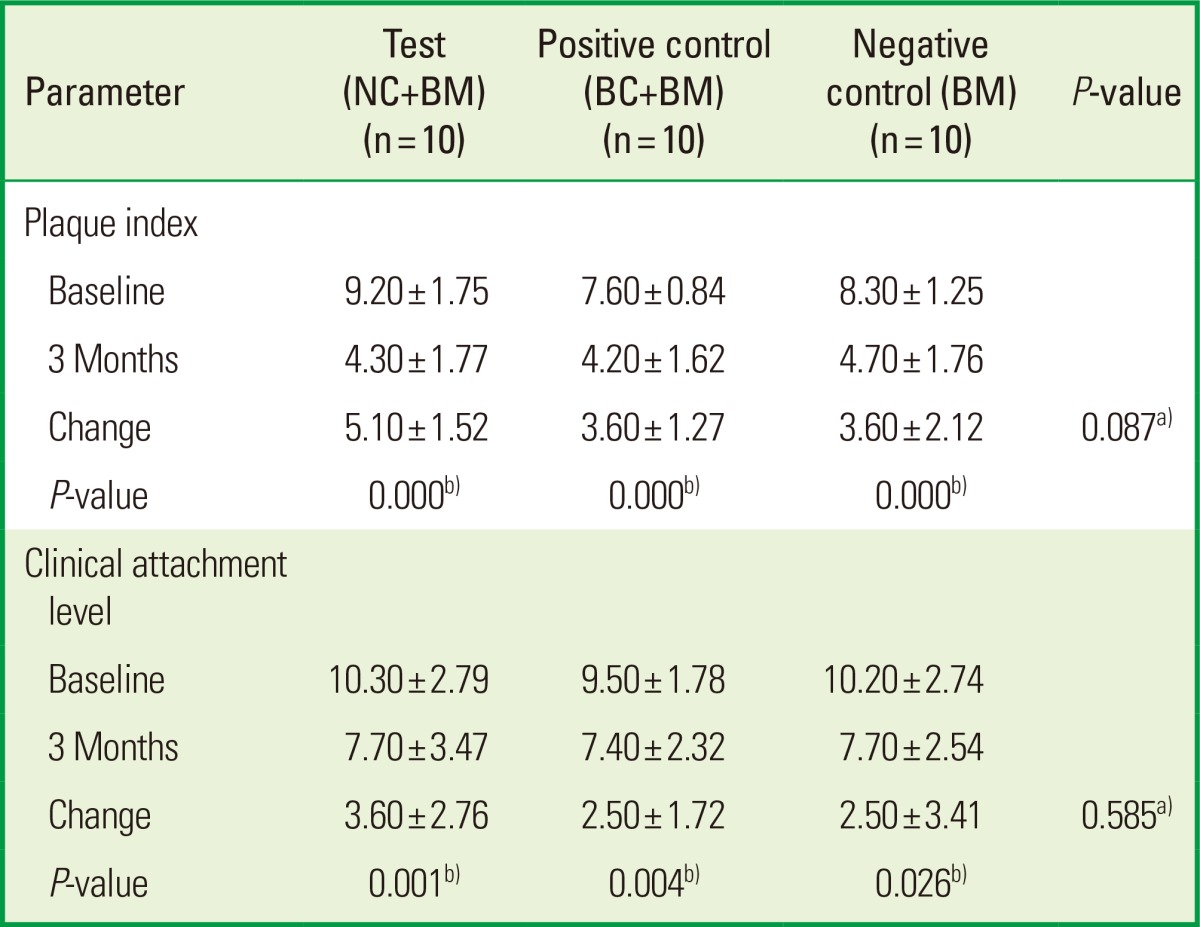
Values are presented as mean±standard deviation.
NC: porous nonchemical cross-linking collagen membrane, BC: bilayer collagen membrane, BM: bone mineral, PD: probing pocket depth, CAL: clinical attachment level.
a)There were no statistically significant differences between the test and control groups.
b)There were statistically significant differences compared to baseline in both test and control groups.
Table 4.
Radiographic bone fill.
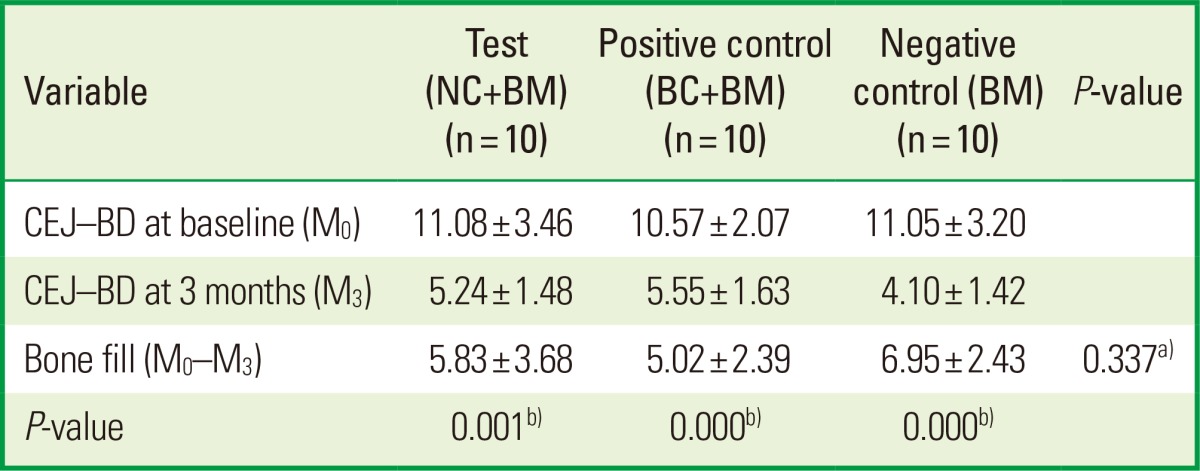
Values are presented as mean±standard deviation.
NC: porous nonchemical cross-linking collagen membrane, BC: bilayer collagen membrane, BM: bone mineral, CEJ-BD: distance between the cemento-enamel junction and the bottom of the defect.
a)There was no statistically significant difference between the test and control groups.
b)There was statistically significant difference compared to baseline in both test and control groups.
Table 5.
Change of radiographic bone level after surgery.
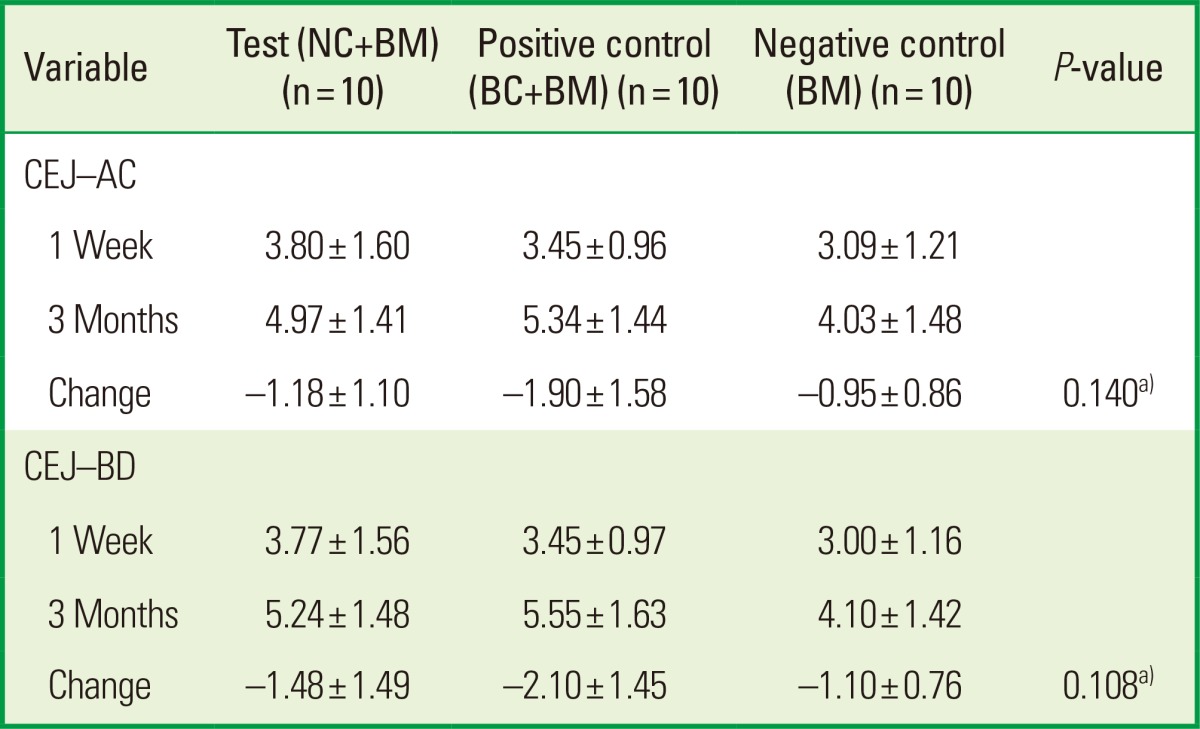
Values are presented as mean±standard deviation.
NC: porous nonchemical cross-linking collagen membrane, BC: bilayer collagen membrane, BM: bone mineral, CEJ-AC: distance between the cemento-enamel junction and the alveolar crest, CEJ-BD: distance between the cemento-enamel junction and the bottom of the defect.
a)There was no statistically significant difference between the test and control groups.
DISCUSSION
In the present study, we clinically assessed and compared the efficacy of GTR therapy using NC collagen and BC collagen membranes in combination with a xenograft for the treatment of intrabony defects. Both collagen membranes (NC and BC) were comparable in terms of pocket depth reduction and gain of CAL. We could not ascertain whether the clinical improvement was due to tissue regeneration, repair, and/or a combination of both healing events because a histological study was not performed. Histometric studies in animals and humans provide evidence that GTR surgery using biodegradable barriers is likely to result in the establishment of a new connective tissue attachment [6,21].
An animal experiment has shown that collagen membranes potentially lead to bone regeneration [22]. Furthermore, human case studies and controlled clinical studies have been presented describing the successful use of collagen membranes in the GBR procedure [23,24]. Various bone graft materials have been used discretely or in combination with GTR to support the regeneration of periodontal tissues. The application of a bone graft produced considerably variable outcomes [25]. In this study, the radiographic bone fill was statistically significant compared to baseline in all treatment groups without a significant difference between the test and control groups. In the evaluation of periodontal regeneration, the formation of new periodontal attachment and the formation of new bone have to be separately evaluated, since they seem to be unrelated events [26]. A GTR device (a porous nonchemical cross-linking membrane) was evaluated previously in one-wall intrabony defects in beagle dogs [17]. In that animal study, new bone height was greater in the membrane-covered group compared to the control group, which did not have a membrane. However, for parameters dealing with the regeneration of periodontal tissue, including junctional epithelium migration and new cementum height, there were no statistically significant differences between the two groups.
The present study demonstrated clinical improvements similar to those reported for several other treatment strategies, including biologic mediators such as platelet derived growth factor, autogenous bone grafts, bone biomaterials, enamel matrix derivates, and GTR as discrete protocols or in combinations [27,28]. The variation in outcomes may be related to defect location and configuration, surgical technique, postsurgery protocol, evaluation method, and the presentation of the data. Only deep intrabony defects seemed to have a substantial clinical regenerative potential in absolute measures [29]. Thus, direct side-by-side comparisons of treatment effects between studies may not be entirely meaningful in the present and other settings.
Wound failure including membrane exposure is a disaster in periodontal-regenerative therapy utilizing the GTR technique because it makes the procedure unpredictable in clinical practice [27,30]. Trombelli et al. [30] demonstrated the clinical significance of wound closure in a retrospective evaluation of GTR therapy. The increase in probing bone level in sites without membrane exposure averaged 4.1±2.3 mm, in contrast to 2.2±2.3 mm in sites with membrane exposure. In this study, complete gingival wound closure was accomplished and membrane exposure was not observed in any cases. The radiographic bone fill was over 5 mm in both the NC and BC groups (Table 4). Bony change indicating resorption of the alveolar crest and grafted BM after GTR/bone graft combination seemed to be independent of the type of the barrier membrane in this study, that is, there was no significant difference in bony change between the NC and BC groups.
Within the limit of this study, NC and BC are comparable in terms of clinical and radiographic outcomes for the treatment of periodontal intrabony defects.
Further investigation is necessary to confirm the influence of NC on periodontal tissue regeneration in light of the small population, short evaluation period, and limited design of this study.
ACKNOWLEDGEMENTS
This study was supported by Wonkwang University in 2014.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Caton J, Nyman S, Zander H. Histometric evaluation of periodontal surgery. II. Connective tissue attachment levels after four regenerative procedures. J Clin Periodontol. 1980;7:224–231. doi: 10.1111/j.1600-051x.1980.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 2.Zucchelli G, Bernardi F, Montebugnoli L, De SM. Enamel matrix proteins and guided tissue regeneration with titanium-reinforced expanded polytetrafluoroethylene membranes in the treatment of infrabony defects: a comparative controlled clinical trial. J Periodontol. 2002;73:3–12. doi: 10.1902/jop.2002.73.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–260. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 4.Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration: animal and human studies. Periodontol 2000. 1993;1:26–35. [PubMed] [Google Scholar]
- 5.Cortellini P, Clauser C, Prato GP. Histologic assessment of new attachment following the treatment of a human buccal recession by means of a guided tissue regeneration procedure. J Periodontol. 1993;64:387–391. doi: 10.1902/jop.1993.64.5.387. [DOI] [PubMed] [Google Scholar]
- 6.Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9:290–296. doi: 10.1111/j.1600-051x.1982.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 7.Stahl SS, Froum S, Tarnow D. Human histologic responses to guided tissue regenerative techniques in intrabony lesions. Case reports on 9 sites. J Clin Periodontol. 1990;17:191–198. doi: 10.1111/j.1600-051x.1990.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 8.Hardwick R, Scantlebury TV, Sanchez R, Whitley N, Ambruster J. Membrane design criteria for guided bone regeneration of the alveolar ridge. In: Buser D, Dahlin C, Schenk RK, editors. Guided bone regeneration in implant dentistry. Chicago: Quintessence; 1994. pp. 101–136. [Google Scholar]
- 9.Haney JM, Nilveus RE, McMillan PJ, Wikesjo UM. Periodontal repair in dogs: expanded polytetrafluoroethylene barrier membranes support wound stabilization and enhance bone regeneration. J Periodontol. 1993;64:883–890. doi: 10.1902/jop.1993.64.9.883. [DOI] [PubMed] [Google Scholar]
- 10.Black BS, Gher ME, Sandifer JB, Fucini SE, Richardson AC. Comparative study of collagen and expanded polytetrafluoroethylene membranes in the treatment of human class II furcation defects. J Periodontol. 1994;65:598–604. doi: 10.1902/jop.1994.65.6.598. [DOI] [PubMed] [Google Scholar]
- 11.Robert PM, Frank RM. Periodontal guided tissue regeneration with a new resorbable polylactic acid membrane. J Periodontol. 1994;65:414–422. doi: 10.1902/jop.1994.65.5.414. [DOI] [PubMed] [Google Scholar]
- 12.Chvapil M. Reconstituted collagen. In: Viidik A, Vuust J, editors. Biology of collagen. London: Academic Press; 1980. pp. 313–325. [Google Scholar]
- 13.Rao KP, Joseph T. Collagen graft copolymers and their biomedical applications. In: Nimni ME, editor. Collagen, vol. III. Biotechnology. Boca Raton, FL: CRC Press Inc.; 1988. pp. 63–86. [Google Scholar]
- 14.Quteish D, Dolby AE. The use of irradiated-crosslinked human collagen membrane in guided tissue regeneration. J Clin Periodontol. 1992;19:476–484. doi: 10.1111/j.1600-051x.1992.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 15.Olde Damink LH, Dijkstra PJ, Van Luyn MJ, Van Wachem PB, Nieuwenhuis P, Feijen J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J Mater Sci Mater Med. 1995;6:460–472. [Google Scholar]
- 16.Rothamel D, Schwarz F, Sculean A, Herten M, Scherbaum W, Becker J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast-like cells. Clin Oral Implants Res. 2004;15:443–449. doi: 10.1111/j.1600-0501.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee CK, Koo KT, Kim TI, Seol YJ, Lee YM, Rhyu IC, et al. Biological effects of a porcine-derived collagen membrane on intrabony defects. J Periodontal Implant Sci. 2010;40:232–238. doi: 10.5051/jpis.2010.40.5.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, et al. Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodontics Restorative Dent. 1998;18:321–331. [PubMed] [Google Scholar]
- 19.Camelo M, Nevins ML, Lynch SE, Schenk RK, Simion M, Nevins M. Periodontal regeneration with an autogenous bone-Bio-Oss composite graft and a Bio-Gide membrane. Int J Periodontics Restorative Dent. 2001;21:109–119. [PubMed] [Google Scholar]
- 20.Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 21.Magnusson I, Batich C, Collins BR. New attachment formation following controlled tissue regeneration using biodegradable membranes. J Periodontol. 1988;59:1–6. doi: 10.1902/jop.1988.59.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Zahedi S, Legrand R, Brunel G, Albert A, Dewe W, Coumans B, et al. Evaluation of a diphenylphosphorylazide-crosslinked collagen membrane for guided bone regeneration in mandibular defects in rats. J Periodontol. 1998;69:1238–1246. doi: 10.1902/jop.1998.69.11.1238. [DOI] [PubMed] [Google Scholar]
- 23.Hammerle CH, Lang NP. Single stage surgery combining transmucosal implant placement with guided bone regeneration and bioresorbable materials. Clin Oral Implants Res. 2001;12:9–18. doi: 10.1034/j.1600-0501.2001.012001009.x. [DOI] [PubMed] [Google Scholar]
- 24.Zitzmann NU, Naef R, Scharer P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int J Oral Maxillofac Implants. 1997;12:844–852. [PubMed] [Google Scholar]
- 25.Trombelli L, Heitz-Mayfield LJ, Needleman I, Moles D, Scabbia A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J Clin Periodontol. 2002;29(Suppl 3):117–135. doi: 10.1034/j.1600-051x.29.s3.7.x. [DOI] [PubMed] [Google Scholar]
- 26.Hugoson A, Ravald N, Fornell J, Johard G, Teiwik A, Gottlow J. Treatment of class II furcation involvements in humans with bioresorbable and nonresorbable guided tissue regeneration barriers. A randomized multi-center study. J Periodontol. 1995;66:624–634. doi: 10.1902/jop.1995.66.7.624. [DOI] [PubMed] [Google Scholar]
- 27.Sanz M, Tonetti MS, Zabalegui I, Sicilia A, Blanco J, Rebelo H, et al. Treatment of intrabony defects with enamel matrix proteins or barrier membranes: results from a multicenter practice-based clinical trial. J Periodontol. 2004;75:726–733. doi: 10.1902/jop.2004.75.5.726. [DOI] [PubMed] [Google Scholar]
- 28.Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 29.Sculean A, Windisch P, Chiantella GC, Donos N, Brecx M, Reich E. Treatment of intrabony defects with enamel matrix proteins and guided tissue regeneration. A prospective controlled clinical study. J Clin Periodontol. 2001;28:397–403. doi: 10.1034/j.1600-051x.2001.028005397.x. [DOI] [PubMed] [Google Scholar]
- 30.Trombelli L, Kim CK, Zimmerman GJ, Wikesjo UM. Retrospective analysis of factors related to clinical outcome of guided tissue regeneration procedures in intrabony defects. J Clin Periodontol. 1997;24:366–371. doi: 10.1111/j.1600-051x.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]