SUMMARY
Objectives
The standard concurrent radiotherapy and chemotherapy regimens for patients with oropharyngeal cancer are highly toxic. Human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC) has recently emerged as a distinct biological and clinical entity with improved response to treatment and prognosis. A tailored therapeutic approach is needed to optimize patient care. The aim of our study was to investigate the impact of HPV and smoking status on early toxicities (primarily mucositis) associated with concurrent chemotherapy and radiotherapy in patients with OPSCC.
Materials and methods
We retrospectively evaluated 72 consecutive patients with OPSCC and known HPV status treated with concurrent radiotherapy and chemotherapy at our institution. Treatment-related toxicities were stratified by smoking and HPV status and compared using univariate and multivariate logistic regression.
Results
HPV-positive patients had a 6.86-fold increase in the risk of having severe, grade 3–4 mucositis. This effect was preserved after adjusting for patient smoking status, nodal stage, radiotherapy technique and radiotherapy maximum dose. Additionally, HPV status had significant effect on the objective weight loss during treatment and at three months after treatment. Consistently, non-smokers had a significant 2.70-fold increase in the risk of developing severe mucositis.
Conclusion
Risk factors for OPSCC modify the incidence of treatment-related early toxicities, with HPV-positive and non-smoking status correlating with increased risk of high grade mucositis and associated outcomes. Retrospective single-institution studies need to be interpreted cautiously. However, this finding is important to consider when designing therapeutic strategies for HPV-positive patients and merits further investigation in prospective clinical trials.
Keywords: Human papillomavirus, Smoking, Oral mucositis, Radiotherapy, Chemotherapy, Squamous cell of head and neck
Introduction
Although alcohol and tobacco use are well characterized risk factors for head and neck cancer, human papillomavirus (HPV)- associated oropharyngeal squamous cell carcinoma (OPSCC) has been identified as a distinct tumor entity with unique biological, pathological and clinical features [1–5]. Approximately 63% of all OPSCC cases diagnosed between 2004 and 2008 were attributable to HPV [6]. In a recent systematic review and meta-analysis, patients with HPV-related OPSCC had a 53% better overall survival (OS) and a 52% better progression-free survival (PFS) vs. those with HPV-unrelated OPSCC [7].
Concurrent chemoradiotherapy (CRT) is a standard definitive treatment for patients with locally advanced head and neck squamous cell carcinoma (HNSCC). Combined treatment leads to substantial acute side effects (e.g., mucositis), resulting in significant short- and long-term morbidity [8]. Given the favorable prognosis of HPV-related OPSCC [9–11], developing alternative strategies for reducing treatment related morbidity is imperative.
Smokers with HNSCC have diffuse alterations in the mucosa that lines their entire throat, while HPV-positive patients and non-smokers tend to have focal genetic alterations [12,13], which may impact the quality of the mucosa and thus lead to differences in tolerance to CRT. There have been no published reports on the role of HPV status in early treatment-related toxicities. Understanding the differential tolerance of normal mucosa in HPV-related vs. -unrelated OPSCC patients is important when considering the risk for treatment-related toxicities and when designing therapeutic strategies more specific for HPV-positive patients.
We hypothesized that patients with OPSCC may have differential mucosal inflammatory response (mucositis) to CRT based on their etiologies (HPV vs. smoking-associated OPSCC). This study aimed to measure the impact of HPV status, smoking, and other covariates on the degree of acute toxicities (primarily mucositis) during and immediately after CRT in a consecutive series of OPSCC patients.
Patients and methods
Study design and eligibility criteria
This retrospective study was conducted at the Comprehensive Cancer Center of Wake Forest University (CCCWFU) and approved by our Institutional Review Board. We identified all consecutive patients with OPSCC in our Cancer Registry treated with definitive concurrent radiotherapy and chemotherapy regimens between January 2007 and September 2012. Only patients with known HPV status were eligible for analysis. Additional criteria for eligibility include: HPV + unknown primary HNSCC, and OPSCC with oligometastatic disease who received standard CRT for their loco-regional disease. We excluded patients treated with adjuvant concurrent CRT, patients not treated at CCCWFU, and/or those with less than three months of follow-up after completing treatment.
Retrospective data collection
Utilizing an electronic medical record (EMR) review, we collected information on: age at diagnosis, sex, race, HPV status, tobacco use, tumor/node/metastasis (TNM) stage, highest grade of mucositis, and presence of excessive mucus production during and immediately after treatment. Patients were considered smokers if they were actively smoking or had more than a ten pack-year history and had quit less than one year before diagnosis. Excessive mucus production was defined as present when specifically described in the notes and/or patients were prescribed medications to address it (scopolamine patch, mucolytics). Mucositis was graded using the Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [14] (Supplementary Table 1). Dysphagia was retrospectively assessed by patient’s ability to take oral nutrition at the end of treatment and by the need for referral for speech and swallowing evaluation and treatment during follow-up. Aspiration was documented either by flexible endoscopic evaluation of swallowing (FEES) or by imaging studies describing aspiration pneumonia in the post-treatment follow-up. We documented patients’ body weights at the beginning, end, and three months after therapy, duration of utilization of a feeding tube, and hospital admissions due to mucositis and related complications. Alcohol use was not well-documented in the EMR and thus was excluded from this analysis. Evidence of treatment efficacy (e.g., response to treatment, disease progression and survival to date) were also documented.
HPV and p16 analyses
HPV status was defined based on polymerase chain reaction (PCR) testing [15–18]. Only patients found HPV-negative or HPV-positive for high-risk serotype were included in our study. We attempted to determine p16 status in all HPV-positive patients; however, only 39 out of 62 patients had sufficient tumor tissue available for p16 testing (data not shown). All but one of the 39 HPV-positive patients were found p16 positive. The patient found p16 negative is a smoker [19]. PCR and p16 procedures are described in the Supplementary Materials section.
Treatment
Radiation was delivered using either 3D conformal radiotherapy (3D-CRT) or intensity-moderated radiation therapy (IMRT). A parotid- sparing approach was used whenever possible. The total treatment dose, oral cavity/oropharynx (OP) maximum (to 1 cc volume) and mean dose were determined based on review of all RT plans when available (data missing in one patient). The oropharynx was defined based on AJCC landmarks and included the pharynx between the soft palate, hyoid inferiorly, and the lateral pharyngeal walls/mucosa. All patients received CRT. The chemotherapy used concurrent with definitive radiotherapy included cisplatin in 58 patients (80.5%); cisplatin and cetuximab (according to Radiation Therapy Oncology Group [RTOG] 0522 [NCT00265941]) in one patient (1.4%); carboplatin and paclitaxel in seven patients (9.7%); and docetaxel in two patients (2.8%). Four patients (5.6%) received induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil (TPF) before the definitive CRT (Supplementary Table 2). Per our Head and Neck Cancer Group policy, prophylactic gastrostomy tube (G-tube) placement was recommended for all patients undergoing CRT.
Statistical analysis
Descriptive statistics were calculated for variables of interest. For continuous measures we used means and standard deviations and for categorical measures we used counts and percents. Etiologic groups (HPV-positive vs. HPV-negative and smoker vs. non-smoker) were compared using Fisher’s exact and Chi-Square tests (when expected cell counts exceeded 5) for categorical outcomes and two-sample t-tests for continuous variables. Odds ratios are presented for primary outcomes. For time to event outcomes, overall survival and progression free survival, Kaplan–Meier curves were estimated and groups were compared using the log rank test. Response rates were also compared between groups using Chi-Square tests.
A series of simple logistic regression models were fit to assess the relationship between each individual variable and mucositis grade (1–2 vs. 3–4). Next, all variables that had at least a modest association with mucositis (i.e. p < 0.20) were considered for a multivariate logistic regression model (MVA). In this MVA, HPV status and smoking status were forced into the model regardless of their statistical significance since these two variables were central to the hypotheses of this paper. Once these variables were included, a forward selection modeling process was used to identify additional variables to include in the MVA using a criteria of p ≤ 0.10 for retaining a variable. In addition, based on the sample size for this study, we determined that at most 5 variables could be retained in the final MVA; thus, if more than five variables were identified in the selection process, then the variables would be removed based on their level of significance until five remained.
All statistical tests were performed using 2-sided tests, and all analyses were performed in SAS, version 9.2 (SAS Institute, Inc.).
Results
Patient characteristics
Seventy-two patients diagnosed and treated at the CCCWFU between January 2007 and September 2012 met eligibility requirements for this analysis. Patient characteristics are described in Table 1. The mean age at diagnosis was 59.5 years (range 39–82 years). Most patients were Caucasian (89%), male (85%), non-smokers (58%), and HPV-positive (79%). Of the eight African Americans, six patients were HPV-negative (p < 0.001) and seven patients were smokers (p = 0.008). As expected, the majority of HPV-negative patients were smokers, while the majority of HPV-positive patients were non-smokers (P = 0.008). HPV-positive patients presented with earlier T stage (68.4% having T1–2 tumors vs 46.7% of HPV-negative patients) but with more advanced nodal disease (87.7% having N2–3 disease compared to 73.4% of HPV-negative patients) (Table 1). The use of IMRT favored the HPV-positive group (82.5% vs. 73.3%). Radiotherapy delivery technique and doses were not significantly different between HPV-positive and negative groups. The mean and max OP dose was equally distributed across HPV-positive and HPV-negative patients. The volume and stage of disease treated was well-balanced between groups. Cisplatin was administered concurrent with radiotherapy in a larger percentage of HPV-positive patients, while a larger percentage of HPV-negative patients received more radiosensitizing regimens such as carboplatin/paclitaxel or docetaxel, as well as induction chemotherapy before CRT (Supplementary Table 2). The median follow-up was 26.4 months.
Table 1.
Patient and treatment characteristics.
| Characteristic | N (%) | HPV− N (%) |
HPV+ N (%) |
P | Non-smoker N(%) |
Smoker N (%) |
P |
|---|---|---|---|---|---|---|---|
| Age in years | |||||||
| Mean (range) | 59.5 (39–82) | 66 (53–82) | 58 (39–78) | 0.002 | 65 (42–82) | 58 (39–78) | 0.30 |
| Sex | |||||||
| Male | 61 (85) | 11 (73.3) | 50 (87.7) | 0.22 | 37 (88.1) | 24 (80) | 0.51 |
| Female | 11 (15) | 4 (26.7) | 7 (12.3) | 5 (11.9) | 6 (20) | ||
| Race | |||||||
| African-American | 8 (11) | 6 (40) | 2 (3.5) | <0.001 | 1 (2.4) | 7 (23.3) | 0.008 |
| Caucasian | 64 (89) | 9 (60) | 55 (96.5) | 41 (97.6) | 23 (76.7) | ||
| Smoking | |||||||
| No | 42 (58) | 4 (26.7) | 38 (69.3) | 0.008 | |||
| Yes | 30 (42) | 11 (73.3) | 19 (30.7) | ||||
| Tumor Stage | |||||||
| Tx | 3 (4) | 0 (0) | 3 (5.3) | 0.002 | 2 (4.8) | 1 (3.3) | 0.54 |
| T1 | 20 (28) | 3 (20) | 17 (29.8) | 13 (30.9) | 7 (23.3) | ||
| T2 | 26 (36) | 4 (26.7) | 22 (38.6) | 15 (35.7) | 11 (36.7) | ||
| T3 | 10 (14) | 0 (0) | 10 (17.5) | 7 (16.7) | 3 (10) | ||
| T4 | 13 (18) | 8 (53.3) | 5 (8.8) | 5 (11.9) | 8 (26.7) | ||
| Nodal Stage | |||||||
| N0 | 3 (4) | 2 (13.3) | 1 (1.7) | 0.41 | 2 (4.8) | 1 (3.3) | 0.62 |
| N1 | 8 (11) | 2 (13.3) | 6 (10.6) | 6 (14.3) | 2 (6.7) | ||
| N2a | 1 (1.4) | 0 (0) | 1 (1.7) | 0 (0) | 1 (3.3) | ||
| N2b | 27 (37.5) | 4 (26.7) | 23 (40.4) | 17 (40.5) | 10 (33.3) | ||
| N2c | 26 (36.1) | 6 (40) | 20 (35.1) | 14 (33.3) | 12 (40.0) | ||
| N3 | 7 (10) | 1 (6.7) | 6 (10.5) | 3 (7.1) | 4 (13.4) | ||
| Metastasis Stage | |||||||
| M0 | 69 (96) | 15 (100) | 54 (94.7) | 0.99 | 42 (100) | 27 (90) | 0.07 |
| M1 | 3 (4) | 0 (0) | 3 (5.3) | 0 (0) | 3 (10) | ||
| Stage Grouping | |||||||
| III | 8 (11) | 1 (6.7) | 7 (12.3) | 0.48 | 6 (14.3) | 2 (6.7) | 0.14 |
| IVa | 53 (74) | 11 (73.3) | 42 (73.7) | 32 (76.2) | 21 (70) | ||
| IVb | 8 (11) | 3 (20) | 5 (8.8) | 4 (9.5) | 4 (13.3) | ||
| IVc | 3 (4) | 0 (0) | 3 (5.2) | 0 (0) | 3 (10) | ||
| HPV Status | |||||||
| Negative | 15 (21) | 4 (8.5) | 11 (36.7) | 0.008 | |||
| Positive | 57 (79) | 38 (91.5) | 19 (63.3) | ||||
| Treatment Paradigm | |||||||
| Definitive CRT | 68 (94.4) | 13 (86.7) | 55 (96.5) | 0.19 | 41 (97.6) | 27 (90) | 0.30 |
| Definitive induction | 4 (5.6) | 2 (13.3) | 2 (3.5) | 1 (2.4) | 3 (10) | ||
| RT Technique | |||||||
| 3DCRT | 14 (19) | 4 (26.7) | 10 (17.5) | 0.47 | 7 (16.7) | 7 (23.3) | 0.55 |
| IMRT | 58 (81) | 11 (73.3) | 47 (82.5) | 35 (83.3) | 23 (76.7) | ||
| RT Dose | |||||||
| Mean (Range) OP(Gy) | 60 (50–66.5) | 59 (50–66) | 63 (50–66.5) | 0.83 | 60 (50–66.5) | 63 (50–66.5) | 0.70 |
| Max (Range) OP(Gy) | 73.5 (70–76) | 73.5 (70–74) | 73.5 (70–75.5) | 0.79 | 73.5 (70–75) | 73.5 (70–75.5) | 0.96 |
| Mucositis | |||||||
| Grade 1 | 1 (1.4) | 1 (6.7) | 0 (0) | 0.005 | 0 (0) | 1 (3.3) | 0.075 |
| Grade 2 | 32 (44.4) | 11 (73.3) | 21 (36.8) | 15 (35.7) | 17 (56.7) | ||
| Grade 3 | 30 (41.6) | 1 (6.7) | 29 (50.9) | 19 (45.2) | 11 (36.7) | ||
| Grade 4 | 9 (12.6) | 2 (13.3) | 7 (12.3) | 8 (19.1) | 1 (3.3) | ||
CRT (chemoradiotherapy); Gy (Gray); HPV (human papillomavirus); IMRT (intensity modulated radiotherapy); IQR (interquartile range); M = metastasis; N = nodal; N = number; OP = oropharyngeal; RT (radiotherapy); T = tumor.
Association of grade 3–4 mucositis with HPV and smoking status: univariate analysis
Grade 1–2 mucositis and grade 3–4 mucositis were grouped for all comparisons due to the inherent quantitative and qualitative limitations of toxicity grading in the retrospective setting. No patients with grade 5 mucositis were identified. A univariate analysis was completed to identify covariates impacting the development of grade 3–4 mucositis and subsequent consideration of inclusion into the multivariate model. Patients with HPV-positive tumors had a 6.86-fold increase in the odds of having grade 3–4 mucositis compared to their HPV-negative counterparts (95% confidence interval [CI], 1.7–27.1) (P = 0.0061). Non-smokers had a 2.70-fold increase in the odds of developing grade 3–4 mucositis compared to smokers (95% CI, 1.03–7.09) (P = 0.044) (Table 2). The absence of smoking was associated with a statistically non-significant increase in grade 3–4 mucositis in the HPV-positive subgroup similar to its effect in the entire study population (P = 0.24) (Table 2). N-stage, but not T-stage, increased the odds of grade 3–4 mucositis (N0-N2a vs. N2b, N2c,-N3: OR 2.8, 95% CI 0.76–10.3; p = 0.122; T3, T4 vs. T0–T1–T2: OR 0.53, 95% CI 0.20–1.45; p = 0.215). Race (OR 0.24, 95% CI 0.046 to 1.299) (p = 0.098) and radiotherapy parameters such as the use of IMRT relative to 3DCRT (OR 3.8, 95% CI 1.07–13.6) (p = 0.0397) and oral cavity max dose (OR 1.06, 95% CI 0.1.0–1.13), (p = 0.064) increased the odds of grade 3–4 mucositis and met criteria for consideration in the multivariate model (data not shown).
Table 2.
Results of univariate and multivariate analyses.
| Mucositis Grade
|
Odds ratio | 95% Confidence interval | P | ||
|---|---|---|---|---|---|
| 1–2 N (%) |
3–4 N (%) |
||||
| Univariate analysis | |||||
| All patients | 33 (43) | 44 (57) | |||
| HPV-positive | 21 (34) | 41 (66) | 6.86 | 1.7–27.1 | 0.0061 |
| HPV-negative | 12 (80) | 36 (20) | |||
| Non-smokers | 15 (36) | 27 (64) | |||
| Smokers | 18 (60) | 12 (40) | 2.70 | 1.03–7.09 | 0.044 |
| HPV-positive patients | |||||
| Non-smokers | 12 (31.6) | 26 (68.4) | |||
| Smokers | 9 (47) | 10 (53) | 1.95 | 0.63–6.04 | 0.24 |
| Multivariate analysis: odds of grade 3–4 mucositis | |||||
| Nodal stage (N0–N2a vs. N2b-N3) | 0.27 | 0.06–1.30 | 0.100 | ||
| IMRT (yes vs. no) | 0.20 | 0.04–0.87 | 0.032 | ||
| Max oropharyngeal radiotherapy dose | 1.08 | 1.00–1.15 | 0.032 | ||
| Smoking (Smokers vs. non-smokers) | 0.52 | 0.16–1.70 | 0.278 | ||
| HPV (Positive vs. negative) | 5.30 | 1.15–24.4 | 0.032 | ||
CI = confidence interval; HPV = human papillomavirus; N = number; N = node.
Predictors of grade 3–4 mucositis: multivariate analysis
Univariate analyses suggested that six possible variables could be included in the multivariate models: HPV status (p = 0.0061), smoking status (p = 0.044), nodal status (p = 0.122), race (p = 0.098), radiation therapy type (p = 0.03), and maximum OP dose (p = 0.06). Once HPV and smoking status were included in the multivariate model, three of these four additional variables met the inclusion criteria (p ≤ 0.10). These were nodal status (p = 0.10), radiation therapy type (IMRT vs 3DCRT, p = 0.032) and intensity (maximum OP dose, p = 0.032). Race did not meet the inclusion criterion (p = 0.78).
The impact of HPV on the likelihood of developing grade 3–4 mucositis was preserved in this multivariate model after adjusting for the relative contributions of smoking, nodal staging, radiation therapy type (IMRT vs 3DCRT) and intensity (maximum OP dose). The adjusted odds ratio for developing grade 3–4 mucositis for HPV-positive patients was 5.3 (p = 0.032) (Table 2). IMRT treatment and OP max dose showed statistically significant impact on the rate of grade 3–4 mucositis in this multivariate model (P = 0.032 for both variables).
Association of weight loss with HPV and smoking status
HPV status had a statistically significant effect on weight loss measured at the end of treatment (mean loss 15.0 lb in the HPV-positive patients vs. 8.3 lb in HPV-negative patients; P = 0.015) and at three months after treatment (mean loss 23.1 lb in the HPV-positive patients vs. 12.6 lb in HPV-negative patients; P = 0.013). A similar difference was noted when measured as a percentage change in pre-treatment body weight (P = 0.045 at the end of treatment and P = 0.096 at 3 months post-treatment). Non-smoking was associated with a non-significant trend towards increased weight loss during (P = 0.21), and at three months post-treatment (P = 0.5) (Table 3).
Table 3.
Correlation of mucositis-associated outcomes with HPV status and smoking.
| HPV+ N = 62 |
HPV− N = 15 |
P | Non-smokers N = 47 |
Smokers N = 30 |
P | |
|---|---|---|---|---|---|---|
| On-therapy mean Δ weight (lb) | −15.0 | −8.3 | −14.8 | −12 | ||
| Δ (95% CI) | 6.72 (1.36–12.1) | 0.015 | 2.9 (1.7–7.4) | 0.21 | ||
| On-therapy mean Δ weight (%) | −7.6 | −4.8 | −7.1 | −6.9 | ||
| Δ (95% CI) | 2.8 (0.06–5.4) | 0.045 | 0.2 (−2.0 to 2.5) | 0.84 | ||
| 3 mo post-therapy mean Δ weight (lb) | −23.1 | −12.6 | −22.0 | −19.5 | ||
| Δ (95% CI) | 10.6 (2.3–18.8) | 0.013 | 2.5 (4.6–9.6) | 0.5 | ||
| 3 mo post-therapy mean Δ weight (%) | −11.6 | −8.2 | −10.8 | −10.9 | ||
| Δ (95% CI) | 3.4 (−0.6 to 7.5) | 0.096 | −0.2 (−3.6 to 3.2) | 0.92 | ||
| Gastrostomy durationa | ||||||
| Mean (SD) | 165.4 (163.4) | 117.1 (98.2) | 0.39 | 167.8 (171) | 143.6 (129.7) | 0.56 |
| Aspirationb | ||||||
| N (%) | 19 (33%) | 7 (47%) | 0.38 | 11 (26%) | 15 (50%) | 0.049 |
| Intervention for dysphagia | ||||||
| N (%) | 32 (56%) | 7 (47%) | 0.57 | 24 (57%) | 15 (50%) | 0.63 |
| Excessive mucus | ||||||
| N (%) | 43 (75%) | 9 (60%) | 0.33 | 35 (83%) | 17 (57%) | 0.017 |
| Oral intakec | ||||||
| N (%) | 37 (65%) | 12 (80%) | 0.36 | 24 (57%) | 25 (83%) | 0.023 |
| Hospitalizationd | ||||||
| N (%) | 7 (12%) | 3 (20%) | 0.42 | 6 (14%) | 4 (13%) | 0.90 |
Δ(change); CI (confidence interval); HPV (human papillomavirus); lb (pounds); mo (month), N (number); SD (standard deviation).
Duration displayed in days. Patients who died with gastrostomy tube and who had residual disease were excluded. Of the remaining patients analyzed, 60 were HPV-positive, 9 were HPV-negative, 24 were smokers, and 45 were non-smokers.
Diagnosed by flexible endoscopic evaluation of swallowing or by computed tomography scan of the chest.
Oral intake tolerated at the end of treatment.
For mucositis or mucositis-associated outcomes.
Other mucositis-associated outcomes
Excessive mucus production during treatment, generally associated with worse mucositis, was found more frequently in non-smokers (P = 0.017). Similarly, the inability to take anything by mouth at the end of treatment was significantly increased in non-smokers (P = 0.023). These outcomes were not statistically significant affected by HPV status.
HPV status did not significantly impact the likelihood of aspiration (P = 0.38); however, smokers were 2.0 times more likely than non-smokers (P = 0.049) to develop aspiration as identified by FEES, CT scan, or both.
Smoking and HPV status had no significant impact on duration of G-tube use. When patients with progressive disease who ultimately died with a G-tube were excluded, we found a clinically meaningful difference of 48.3 days in the average duration of G-tube use in HPV-positive vs. HPV-negative patients (P = 0.39). Smoking and HPV status also had no significant effect on hospitalization for mucositis or mucositis-associated complications; or the need for referrals for swallowing-related evaluation and management (Table 4).
Table 4.
Correlation between treatment efficacy and HPV status.
| Outcomes | Overall population N (%) |
HPV+ N (%) |
HPV− N (%) |
P |
|---|---|---|---|---|
| Complete response | 60 (83.3) | 52 (91.2) | 8 (53.3) | 0.002 |
| Partial response | 9 (12.5) | 4 (7.0) | 5 (33.3) | |
| Stable disease | 3 (4.2) 1(1.8) | 2 (13.3) | ||
| Overall population (%) | HPV+ (%) | HPV− (%) | P (%) | |
|
|
||||
| OS at 1 year | 93 | 98 | 71 | 0.0001 |
| OS at 2 years | 85 | 94 | 46 | |
| PFS at 1 year | 89 | 96 | 59 | 0.0008 |
| PFS at 2 years | 86 | 93 | 59 | |
HPV (human papillomavirus); N (number); OS (overall survival); PFS (progression-free survival).
Treatment compliance and outcomes
Of the 72 treated patients, 9 HPV-positive (15.8%) and two HPV-negative (13.3%) patients had either a reduction in the number of cycles or changes in the type and dose of their planned chemotherapy due to severe mucositis. The compliance with the prescribed radiotherapy was high, with only five of 72 patients missing 2–6 Gray (Gy) of their prescribed dose and ten patients having non-significant, one- to four-day interruptions in their radiotherapy (data not shown).
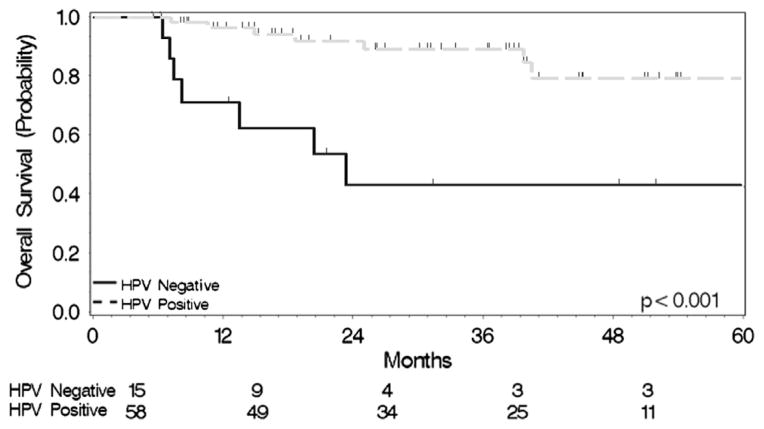
Outcomes were excellent; with 83.3% of patients achieving a complete response, 12.5% a partial response, and 4.2% had stable disease (Table 4). HPV-positive patients were much more likely to achieve a complete response (91.2 vs. 53.3%) and the HPV-negative patients were more likely to obtain a partial response (33.3% vs. 7%) or stable disease (13.3% vs. 1.8%) (P = 0.002). Fourteen of the 72 patients died before the last follow-up, with one- and two-year survival rate estimates of 93% and 85%, respectively. The differences in HPV-positive vs. negative patients again reached statistical significance: 98% vs. 71% and 94 vs. 46% (Log-Rank Chi-Square 14.45, P = 0.0001), respectively (Fig. 1).
Figure 1.
Overall survival stratified by HPV status.
Twelve patients had progressive disease at last follow-up. The one- and two-year progression-free survival (PFS) rates were 89% and 86%, respectively. PFS was significantly different across groups when stratified by HPV status, with one- and two-year PFS rates of 59% and 59% in HPV-negative patients and 96% and 93% in HPV-positive patients (log-rank Chi-Square 11.21, P = 0.0008) (Fig. 2).
Figure 2.
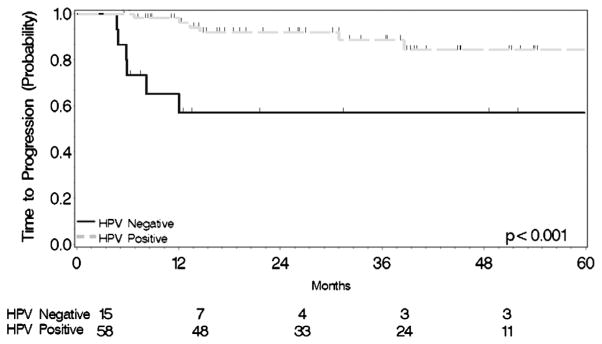
Progression-free survival stratified by HPV status.
Discussion
This single-institution experience with treatment-related mucositis and its relationship to HPV and smoking status revealed a distinct association between HPV status and the predisposition toward aggravated mucositis. Smoking status was initially expected to be a potential confounder in the analysis, but after stratification, the effect of HPV status was well-preserved. After adjusting for both patient- and treatment-related factors, the impact of HPV status on grade 3–4 mucositis was also preserved.
Our analysis shows that HPV status has a well-defined impact not only on subjective determinants of acute toxicity (e.g., an almost sevenfold increase in the odds of developing grade 3–4 mucositis), but also on objective measurements of nutritional status. HPV-positive patients lost both more absolute weight and as a percentage of their initial weight than their HPV-negative counterparts during treatment and at 3 months after treatment, although only the absolute difference in weight was statistically significant at the second timepoint. The effect of mucositis on weight loss despite prophylactic PEG tube use is most likely related to the appetite-suppressive effect of pain and nausea associated with high-grade mucositis. While a similar trend was noted across smoking status, the magnitude was muted relative to the difference across HPV-positive and negative patients.
Concordantly, non-smokers who were predominantly HPV-positive (91.5%), showed a significant increase in the incidence of high-grade mucositis during treatment. This was supported by the association with more objective measurements, such as increased mucus production and inability to take anything by mouth at the end of treatment. This finding contradicts a previous report that examined the impact of active smoking during treatment on mucositis [20]. We lacked adequate documentation of active smoking during treatment in our EMR, and thus we did not include that factor in analysis. In addition, with relatively few patients continuing to smoke during treatment when adequately supported, we considered that history of smoking would be a more influential factor in our analysis.
Radiotherapy parameters were highly influential on the rate of grade 3–4 mucositis. The maximum and mean OP dose was not disproportionately distributed across groups (Table 1), but instead exhibited a strong effect on the risk of mucositis. Contrary to radiotherapy dose, more conformal therapy with IMRT was disproportionately utilized in the HPV-positive group. However, this did not result in a reduction in grade 3–4 mucositis, as would be expected in the setting of similar sensitivity to radiation dose. This highlights the observed interaction between HPV status and sensitivity to high-grade mucositis.
The current thought is that the actual incidence of grade 3–4 mucositis is deterministic (as opposed to stochastic), and depends on a threshold dose of ~30 Gy, although our data argue that beyond an absolute threshold there is a dose-dependent effect for every unit-dose prescribed [21]. Toxicity is also a function of the volume treated. Whereas N2–3 disease was present in 14.3% more of the patients in the HPV-positive subgroup, radiotherapy volumes did not differ significantly, because we routinely treat the same volume of the neck and retropharyngeal lymph nodes in Stage 3 and above patients regardless of extent of nodal status. At the same time, smaller T1–2 tumors were significantly more frequent in the HPV-positive subgroup. Therefore, more research into the effects of radiation dose and volume and their effects on mucositis would be useful.
The difference in acute toxicity noted in the present study was limited to non-life threatening conditions and thus did not lead to an increase in hospitalizations. It is more common to for these patients to receive more aggressive supportive care measures (i.e. intravenous fluids and pain, nausea and nutrition management) as outpatients. As such, adverse symptomatology is more likely to impact quality of life. Other studies have documented a more pronounced effect of CRT on quality of life in HPV-positive vs. HPV-negative patients [22].
The mechanistic factors that contribute to increased treatment-related mucositis in HPV-positive patients are unclear. Factors believed to be involved in a better response to treatment among HPV-positive patients (e.g., immune surveillance to viral-specific tumor antigens, an intact apoptotic response, absence of field cancerization), might facilitate an increased inflammatory response to chemotherapy and radiotherapy inside the tumor and in the surrounding mucosa, resulting in increased mucositis [12,23]. Because HPV-positive patients tend to be non-smokers (70% in our population), they lack mucosal alterations associated with long-term inflammatory insults from tobacco exposure.
The significant finding that smokers had twice the risk of aspiration compared to non-smokers when followed post-treatment, correlates with increased dysphagia in smokers after concurrent CRT reported by other studies [24]. While dysphagia during treatment can correlate with the grade of mucositis, its persistence in the post-treatment period has added complex mechanisms. Chronic alterations of the structure and function of the throat and lung mucosa, as well as alterations in the clearing function and motility of the tracheo-bronchial tree in smokers, can explain the findings of increased aspiration.
As expected, HPV-positive patients showed a better response to treatment and survival compared to HPV-negative patients. Because HPV status is tightly linked to prognosis, research is seeking to de-escalate therapy and thus minimize both acute and longterm toxicity [25]. Modifications in radiotherapy dose are the subject of a phase II study (NCT 01530997), which offers IMRT 54 to 60 Gy concurrent with weekly cisplatin followed by a biopsy of the primary site and neck dissection of lymph nodes that were positive pre-treatment. An alternative approach to reduce treatment dose in the definitive treatment setting may include the use of induction chemotherapy. With excellent response rates to TPF [26,27], induction chemotherapy may be beneficial for patients with a large volume of disease and may reduce the RT dose delivered to critical structures. This approach is the focus of an ongoing phase II Eastern Cooperative Oncology Group trial (NCT01084083) which recently completed accrual (results pending). In addition, RTOG is currently evaluating the efficacy of RT concurrent with cetuximab compared to traditional CRT in HPV-positive patients (NCT01302834). Quality of life and short-term toxicities are secondary endpoints in these trials, and the relationship of their results to classical clinical endpoints (such as PFS and OS) could have wide-reaching impact.
The limitations of our single-site study include accuracy of mucositis grading taken from the EMR and the limited number of patients with HPV-negative tumors. Prospectively-gathered mucositis grading data in a clinical trial would minimize potential for inaccuracy. The rise in incidence of HPV-positive tumors, and the shift in the general epidemiology of the disease, make true matched comparisons to HPV-negative patients challenging. However, with a large patient series, such comparisons would be possible.
Although grade 3–4 mucositis is short-lived, it could have longterm and clinically meaningful implications on patients’ quality of life. Treatment breaks due to mucositis and associated poor nutritional status have hindered cure rates in cases of definitive CRT in multiple disease sites [8,28]. Follow-up studies to evaluate impact of HPV on treatment-related late toxicities are warranted.
Conclusion
In conclusion, our retrospective analysis identified HPV status as an independent predictor of CRT-related grade 3–4 mucositis. Importantly, HPV status had a well-defined impact on objective measurements of nutritional status; HPV positivity adversely impacted patients’ absolute and percent weight loss both during and immediately following treatment. Prospective studies with standardized mucositis grade assessment are needed to confirm the impact and potential mechanisms of the interaction between HPV status and predisposition to high-grade mucositis during CRT. Secondary analyses of recent large prospective studies may be instructive for this purpose.
Supplementary Material
Acknowledgments
This work was supported in part by the Wake Forest Translational Science Institute KL2 Research Scholar program. Biostatistical services were provided by the Comprehensive Cancer Center of Wake Forest University NCI CCSG P30CA012197 grant.
The authors also thank Megan J. Whelen, MPH (supported by the Comprehensive Cancer Center of Wake Forest University) for copyediting, editorial and production assistance and Karen Potvin Klein, MA, ELS (supported by the Biomedical Research Services Administration, Wake Forest School of Medicine) for copyediting and editorial assistance; Susan Butler, PhD (Department of Otolaryngology, Wake Forest School of Medicine) for her contribution to the study concepts and manuscript review; and Amy Franklin, PA-C (Section on Hematology and Oncology, Wake Forest School of Medicine) for her contribution to data collection and manuscript review. The authors also thank the Department of Pathology of Wake Forest School of Medicine for financial support, the Wake Forest School of Medicine Molecular Diagnostics Lab for performing the IHC and ISH, and Access Genetics for interpretation of the HPV genotyping.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.oraloncology.2014.06.010.
Footnotes
Conflict of interest
There is no conflict of interest to declare.
Contributor Information
M. Vatca, Email: mvatca@wakehealth.edu.
J.T. Lucas, Jr., Email: jolucas@wakehealth.edu.
J. Laudadio, Email: jlaudadio@uams.edu.
R.B. D’Agostino, Email: rdagosti@wakehealth.edu.
J.D. Waltonen, Email: jwaltone@wakehealth.edu.
C.A. Sullivan, Email: csulliva@wakehealth. edu.
R. Rouchard-Plasser, Email: rplasser@wakehealth.edu.
M. Matsangou, Email: mmatsang@wakehealth.edu.
J.D. Browne, Email: jdbrowne@wakehealth.edu.
K.M. Greven, Email: kgreven@wakehealth.edu.
M. Porosnicu, Email: mporosni@wakehealth.edu.
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:6758–62. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 5.Psyrri A, Gouveris P, Vermorken JB. Human papillomavirus-related head and neck tumors: clinical and research implication. Curr Opin Oncol. 2009;21:201–5. doi: 10.1097/cco.0b013e328329ab64. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Human papillomavirus– associated cancers—United States, 2004–2008. MMWR. 2012;61:258–261. [PubMed] [Google Scholar]
- 7.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48(12):1191–201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–62. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 11.Rischin D, Young R, Fisher R, Fox SB, Le Qt, Peters LJ, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02 02 phase III trial. J Clin Oncol. 2010;24(27):4142–8. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braakhuis BJ, Tabor MP, Leemans CR, van der Waal I, Snow GB, Brakenhoff RH. Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck. 2002;24(2):198–206. doi: 10.1002/hed.10042. [DOI] [PubMed] [Google Scholar]
- 13.Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–92. [PubMed] [Google Scholar]
- 14.National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0. Bethesda, MD: National Cancer Institute; 2006. [accessed on 21.08.2013]. < http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf>. [Google Scholar]
- 15.de Roda Husman AM, Walboomers JM, van den Brule AJ, Snijders PJ, Meijer CJ, Walboomers JM. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–62. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 16.Nobre RJ, de Almeida LP, Martins TC. Complete genotyping of mucosal human papillomavirus using a restriction fragment length polymorphism analysis and an original typing algorithm. J Clin Virol. 2008;42(1):13–21. doi: 10.1016/j.jcv.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121(11):2465–72. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 18.Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Lo Muzio L, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta-analysis (1988 to 2007) Ann Oncol. 2008;19(10):1681–90. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 19.Yanagawa N, Tamura G, Oizumi H, Takahashi N, Shimazaki Y, Motoyama T. Frequent epigenetic silencing of the p16 gene in non-small cell lung cancers of tobacco smokers. Jpn J Cancer Res. 2002;93(10):1107–13. doi: 10.1111/j.1349-7006.2002.tb01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen AM, Chen LM, Vaughan A, Sreeraman R, Farwell DG, Luu Q, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79(2):414–9. doi: 10.1016/j.ijrobp.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Narayan S, Lehmann J, Coleman MA, Vaughan A, Yang CC, Enepekides D, et al. Prospective evaluation to establish a dose response for clinical oral mucositis in patients undergoing head-and-neck conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(3):756–62. doi: 10.1016/j.ijrobp.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Méndez E, Yueh B, et al. Human papillomavirus-positive oral cavity and oropharyngeal cancer patients do not have better quality-of-life trajectories. Otolaryngol Head Neck Surg. 2012;146(5):739–45. doi: 10.1177/0194599811434707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeer DW, Spanos WC, Vermeer PD, Bruns AM, Lee KM, Lee JH. Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of HPV+ cancer. Int J Cancer. 2013;133:120–9. doi: 10.1002/ijc.28015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng FY, Kim HM, Lyden TH, Haxer MJ, Worden FP, Feng M, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28(16):2732–8. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehanna H, Olaleye O, Licitra L. Oropharyngeal cancer – is it time to change management according to human papilloma virus status? Curr Opin Otolaryngol Head Neck Surg. 2012;20:120–4. doi: 10.1097/MOO.0b013e3283509735. [DOI] [PubMed] [Google Scholar]
- 26.Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–7. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 28.Ang KK, Trotti A, Brown BW, Garden AS, Foote RL, Morrison WH, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):571–8. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.