Abstract
Type II arabinogalactan (AG-II) is a suitable carbohydrate source for Bifidobacterium longum subsp. longum, but the degradative enzymes have never been characterized. In this study, we characterized an exo-β-1,3-galactanase, BLLJ_1840, belonging to glycoside hydrolase family 43 from B. longum subsp. longum JCM1217. The recombinant BLLJ_1840 expressed in Escherichia coli hydrolyzed β-1,3-linked galactooligosaccharides but not β-1,4- and β-1,6-linked galactooligosaccharides. The enzyme also hydrolyzed larch wood arabinogalactan (LWAG), which comprises a β-1,3-linked galactan backbone with β-1,6-linked galactan side chains. The kcat/Km ratio of dearabinosylated LWAG was 24-fold higher than that of β-1,3-galactan. BLLJ_1840 is a novel type of exo-β-1,3-galactanase with a higher affinity for the β-1,6-substituted β-1,3-galactan than for nonsubstituted β-1,3-galactan. BLLJ_1840 has 27% to 28% identities with other characterized exo-β-1,3-galactanases from bacteria and fungi. The homologous genes are conserved in several strains of B. longum subsp. longum and B. longum subsp. infantis but not in other bifidobacteria. Transcriptional analysis revealed that BLLJ_1840 is intensively induced with BLLJ_1841, an endo-β-1,6-galactanase candidate, in the presence of LWAG. This is the first report of exo-β-1,3-galactanase in bifidobacteria, which is an enzyme used for the acquisition of AG-II in B. longum subsp. longum.
INTRODUCTION
Type II arabinogalactan (AG-II) chains are the predominant carbohydrate component of the arabinogalactan protein (AGP), which comprises a β-1,3-linked galactan backbone with β-1,6-linked galactan side chains. In addition, the side chains are substituted with l-arabinose, l-rhamnose, l-fucose, glucuronic acid, and 4-O-methyl-glucuronic acid. Exo-β-1,3-galactanase (EC 3.2.1.145) and endo-β-1,6-galactanse (EC 3.2.1.164) are major degradation enzymes of AG-II. In particular, exo-β-1,3-galactanase hydrolyzes the β-1,3-galactan backbone, bypassing β-1,6-galactan side chains, and consequently releases galactose, β-1,6-galactooligosaccharides, and their derivatives (1, 2). Exo-β-1,3-galactanases, which belong to glycoside hydrolase family 43 (GH43), have been cloned and characterized from several sources, including bacteria and fungi (1, 3–7).
AGP is a complex proteoglycan found in the plant cell wall and is isolated from vegetal products, such as soybean seed, radish root, wheat grain, red wine, and instant coffee (8–12). Larch wood arabinogalactan (LWAG) and gum arabic, which contain AG-II, are used as dietary supplements. In particular, gum arabic has prebiotic effects to increase bifidobacteria in the human body (13, 14). Bifidobacterium longum subsp. longum and B. adolescentis are the primary bifidobacterial species in the gastrointestinal tract of human adults. Unlike with other species of bifidobacteria, these fibers can be fermented by several strains of B. longum subsp. longum and B. adolescentis (15). Because AG-II is a complex carbohydrate with a high molecular mass, these species need to be equipped with a set of glycoside hydrolases for assimilation. In the Carbohydrate-Active Enzymes (CAZy) database (http://www.cazy.org), these species have been shown to encode several genes involved in the degradation of plant carbohydrate polymers, including α-l-arabinofuranosidase (GH43 and GH51) and β-galactosidase (GH42). In addition, the AG-II degradative activity in B. longum subsp. longum increased in the presence of AG-II and was repressed by the addition of glucose (16). However, the major degradative enzymes, exo-β-1,3-galactanase and endo-β-1,6-galactanase, have never been found in bifidobacteria.
We previously characterized the novel GH101 endo-α-N-acetylgalactosaminidase (BLLJ_0168) (17) and GH121 β-l-arabinobiosidase (BLLJ_0212) (18) from B. longum subsp. longum JCM1217. BLLJ_0168 and BLLJ_0212 contain the bifidobacterial membrane-anchoring region. BLLJ_1840 and BLLJ_1841 had low homologies in the N terminus with characterized GH43 exo-β-1,3-galactanases (27% to 28% identities) and GH30 endo-β-1,6-galactanases (20% to 24% identities), respectively. In addition, these proteins contained C-terminal membrane-anchoring regions similar to those of BLLJ_0168 and BLLJ_0212. We predicted that BLLJ_1840 and BLLJ_1841 are AG-II degradative enzymes which localize onto the bifidobacterial surface. In this study, we cloned and characterized a GH43 exo-β-1,3-galactanase, BLLJ_1840, from B. longum subsp. longum JCM1217. This is the first report to discover a candidate enzyme for the degradation of AG-II in B. longum subsp. longum.
MATERIALS AND METHODS
Materials.
LWAG was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). p-Nitrophenyl (pNP) substrates and gum arabic were obtained from Sigma-Aldrich (St. Louis, MO). Potato galactan and laminarin were obtained from Megazyme (Wicklow, Ireland). Partially dearabinosylated gum arabic and LWAG were prepared by mild acid degradation, as described by Ling et al. (19). Extensin was prepared as previously described (18). β-1,3-Galactan was prepared from LWAG by twice-repeated Smith degradation, as described by Tsumuraya et al. (20). β-1,3-Galactooligosaccharides were prepared from β-1,3-galactan by mild acid degradation as described by Sakamoto et al. (21). β-1,4-Galactooligosaccharides were prepared from potato galactan by the use of recombinant endo-β-1,4-galactanase (EC 3.2.1.89) from Bacillus subtilis expressed in Escherichia coli (K. Fujita, unpublished data). β-1,6-Galactooligosaccharides were prepared from dearabinosylated LWAG by the use of recombinant exo-β-1,3-galactanase (BLLJ_1840), as described below. The β-1,3-, β-1,4-, and β-1,6-glalactooligosaccharides were separated on a Bio-Gel P-2 column (25 mm [internal diameter] by 830 mm; Bio-Rad Laboratories, Hercules, CA) equilibrated with distilled water. The oligosaccharides were further purified by high-pressure liquid chromatography on a Cosmosil Sugar-D column (10 mm [internal diameter] by 250 mm; Nacalai Tesque Inc., Kyoto, Japan) at 30°C with a mobile phase of acetonitrile and water (65:35, vol/vol) and a constant flow rate (4.7 ml/min). The elution was monitored by use of a refractive index (RI) detector (Tosoh Corp., Tokyo, Japan), and the fractions that contained the oligosaccharides were collected and examined using sugar composition analysis as previously described (18), matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Leipzig, Germany), and enzymatic digestibility analysis using endo-β-1,6-galactanase, α-l-arabinofuranosidase, and β-galactosidase. Recombinant endo-β-1,6-galactanase (BLLJ_1841) from B. longum subsp. longum was expressed in E. coli (Fujita, unpublished). α-l-Arabinofuranosidase (EC 3.2.1.55) and β-galactosidase (EC 3.2.1.23) from Aspergillus niger were obtained from Megazyme. Other chemicals were obtained from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Expression and purification of recombinant BLLJ_1840.
The genomic DNA of B. longum subsp. longum JCM1217 was extracted using a FastPure DNA kit (TaKaRa, Shiga, Japan) and then used for PCR amplification of the BLLJ_1840 gene. The forward primer (5′-AGGAGATATACCATGGCAGGTGTGGATTACCTG-3′) and the reverse primer (5′-TGCTCGAGTGCGGCCGCTGCGCGGCGTTTGCG-3′) were designed from BLLJ_1840 nucleotides 100 to 117 and 3820 to 3834, respectively. The underlined nucleotides represent nucleotides complementary to the template. The PCR amplification of BLLJ_1840-NΔ32, which encodes amino acids (aa) 33 to 1464, was designed to eliminate the N-terminal signal peptide. Then, the amplicon was cloned into the pET-23d vector (Novagen, Madison, WI) with an In-Fusion Advantage PCR cloning kit (Clontech Laboratories Inc., Palo Alto, CA). The resulting pET23d-BLLJ_1840-NΔ32 plasmid was sequenced on an ABI 3100 DNA sequencer using a BigDye Terminator (version 3.1) cycle sequencing kit (Applied Biosystems, Foster City, CA). The plasmid was transformed into E. coli BL21(λDE3) cells and then grown at 20°C by using an Overnight Express autoinduction system (Novagen). The cell cultures were subsequently centrifuged, and then the pellets were resuspended in BugBuster protein extraction reagent (Novagen). The His-tagged proteins were purified on a Talon metal affinity resin (Clontech Laboratories Inc.) and then desalted and concentrated by using a 10-kDa-cutoff ultrafiltration membrane (Millipore Co., Billerica, MA).
Deletion mutagenesis.
A KOD-Plus mutagenesis kit (Toyobo Co., Ltd., Osaka, Japan) was used to create the BLLJ_1840 deletion mutants using the primers shown in Table S1 in the supplemental material. The primers NΔ reverse and NΔ42 forward were used for the construction of BLLJ_1840-NΔ42 (aa 43 to 1278). The C-terminal deletion mutants were created using plasmid pET23d-BLLJ_1840-NΔ42 as the template and CΔ forward and CΔ reverse primers for NΔ42CΔ257 (aa 43 to 1021), NΔ42CΔ457 (aa 43 to 821), and NΔ42CΔ762 (aa 43 to 516). These mutant enzymes were expressed and purified by using the same procedure used for the BLLJ_1840-NΔ32 enzyme.
Enzyme assays.
The hydrolytic activities of the BLLJ_1840 enzymes were assayed using 1.0% (wt/vol) dearabinosylated LWAG as a standard substrate in 200 μl of 50 mM sodium acetate buffer (pH 5.5). The enzymatic activities were determined as the increase in the amount of the reducing sugars by the Somogyi-Nelson method (22). One unit of enzyme activity toward polysaccharides was defined as the amount of enzyme required to produce 1 μmol of reducing sugars per minute.
The substrate specificity toward polysaccharides was determined at 40°C for the appropriate reaction time with 2.85 milliunits/ml of BLLJ_1840-NΔ42CΔ257 and 1.0% substrates in 200 μl of 50 mM sodium acetate buffer (pH 5.5). The samples were analyzed by the Somogyi-Nelson method (22).
The hydrolytic activity toward β-1,3-galactooligosaccharides was analyzed as follows: the 40-μl reaction mixture contained 50 mM sodium acetate buffer (pH 5.5), 0.1 mM substrate, and 2.85 milliunits/ml of BLLJ_1840-NΔ42CΔ257. After incubating the reaction mixture at 40°C for 120 min, the reaction was stopped by 10 μl of 500 mM NaOH. The amount of liberated galactose was quantified by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) using a galactose standard. The oligosaccharides were analyzed with a CarboPac PA-1 column (4 mm [internal diameter] by 250 mm; Dionex Corp., Sunnyvale, CA). The column was eluted at a flow rate of 1.0 ml/min using the following gradient: 0 to 5 min, 100% eluent A (0.1 M NaOH); 5 to 30 min, 0% to 100% eluent B (0.5 M sodium acetate and 0.1 M NaOH); and 30 to 35 min, 100% eluent B. One unit of enzyme activity toward oligosaccharides was defined as the amount of enzyme required to produce 1 μmol of liberated galactose per minute.
The substrate specificity toward β-1,3-, β-1,4-, and β-1,6-galactooligosaccharides and pNP substrates was analyzed as follows: substrates were incubated at 40°C for 16 h with 2.85 milliunits/ml of BLLJ_1840-NΔ42CΔ257 in 40 μl of 50 mM sodium acetate buffer (pH 5.5). The reaction products were spotted on a silica gel 60 aluminum plate (Merck, Darmstadt, Germany) using a 7:1:2 (vol/vol/vol) n-propanol–ethanol–water solvent mixture. The sugars were visualized by spraying orcinol-sulfate reagent on the plate (23).
The pH dependence of the enzyme activity was determined using dearabinosylated LWAG as the substrate between pH 3.5 and 8.0 in the following buffers: 50 mM sodium acetate (pH 3.5 to 6.0), 50 mM MES (morpholineethanesulfonic acid; pH 5.5 to 7.0), 50 mM HEPES (pH 7.0 to 8.0), and 50 mM Tris-HCl (pH 7.5 to 9.0). The effect of temperature on enzyme activity was examined using 50 mM sodium acetate buffer (pH 4.5) at 15 to 50°C.
Time course of polysaccharide hydrolysis.
The time course of polysaccharide degradation was analyzed by incubating 1.0% dearabinosylated LWAG, LWAG, and β-1,3-galactan with 2.85 milliunits/ml of BLLJ_1840-NΔ42CΔ257 in 200 μl of 50 mM sodium acetate buffer (pH 5.5) at 40°C for up to 320 min. The reaction was stopped by boiling for 3 min, and the reaction mixture was analyzed by HPAEC-PAD as described above.
Kinetic analysis.
The kinetic parameters of BLLJ_1840-NΔ42CΔ257 were determined using 0 to 15 mg/ml dearabinosylated LWAG, 0 to 75 mg/ml LWAG, and 0 to 30 mg/ml β-1,3-galactan as the substrates. In the case of dearabinosylated LWAG, the 200-μl reaction mixture contained 50 mM sodium acetate buffer (pH 5.5), and 2.85 milliunits/ml of BLLJ_1840-NΔ42CΔ257 was incubated at 40°C for 20 min. In the case of LWAG and β-1,3-galactan, the reaction was stopped after a 300-min incubation. The samples were analyzed by the Somogyi-Nelson method as described above.
Culture conditions and RNA extraction.
B. longum subsp. longum JCM1217 was cultured at 37°C under anaerobic conditions using an Anaero-Pack system (Mitsubishi Gas Chemical, Tokyo, Japan) on peptone-yeast extract-Fildes (PYF) medium (24) containing 1.0% l-arabinose, glucose, galactose, β-1,6-galactobiose (Gal2), β-1,6-galactotriose (Gal3), or LWAG. Growth profiles were monitored by measuring the optical density at 600 nm (OD600). Cells were harvested at end exponential growth phase and stored at −80°C until use. The bacterial pellet from 500 μl of culture was disrupted with 200 μl of cell lysis buffer as described by Savard and Roy (25). Total RNA was extracted by use of a NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany). Reverse transcription was performed using RevaTra Ace reverse transcriptase (Toyobo Co., Ltd.).
Quantitative real-time PCR.
Primers were designed using Primer Express software (Applied Biosystems). A BLLJ_0405-encoding RNA polymerase β subunit (rpoB) was used as a reference gene for the normalization of the target genes. The primers for the analysis of rpoB, BLLJ_1840, and BLLJ_1841 are shown in Table S1 in the supplemental material. Real-time PCR was performed on a StepOne Plus system (Applied Biosystems) with SYBR Premix Ex Taq II (TaKaRa) according to the following temperature protocol: 95°C for 30 s and 40 cycles at 95°C for 5 s and 60°C for 30 s. After the last cycle, the melting curve analysis was performed to check the specificity of the PCR. Data were collected and analyzed using StepOne software (version 2.2.2; Applied Biosystems). Cycle threshold values were automatically calculated by the software. Standard curves were generated using plasmid DNA containing the appropriate template inserts.
Assays of bacterial enzyme activities.
B. longum subsp. longum JCM1217 was grown on PYF medium containing 1.0% l-arabinose, galactose, gum arabic, LWAG, or extensin at 37°C for 16 h under anaerobic conditions. The cell cultures were centrifuged at 17,000 × g for 20 min, and the resultant pellets were washed with 50 mM sodium acetate buffer (pH 6.0). Afterwards, the pellets were sonicated with a Branson Sonifier 250 (Danbury, CT). After centrifugation at 17,000 × g for 10 min, the sonicated cell pellets were resuspended with 50 mM sodium acetate buffer (pH 6.0). The sonicated cell suspensions were incubated with β-1,3-Gal3 and β-1,6-Gal3 at 40°C for 16 h and then analyzed by thin-layer chromatography (TLC) and HPAEC-PAD as described above.
Nucleotide sequence accession number.
The BLLJ_1840 nucleotide sequence reported in this study is deposited in the third party annotation section of the DDBJ/GenBank/EBI Data Bank with accession number BR001188.
RESULTS
Sequence analysis of BLLJ_1840.
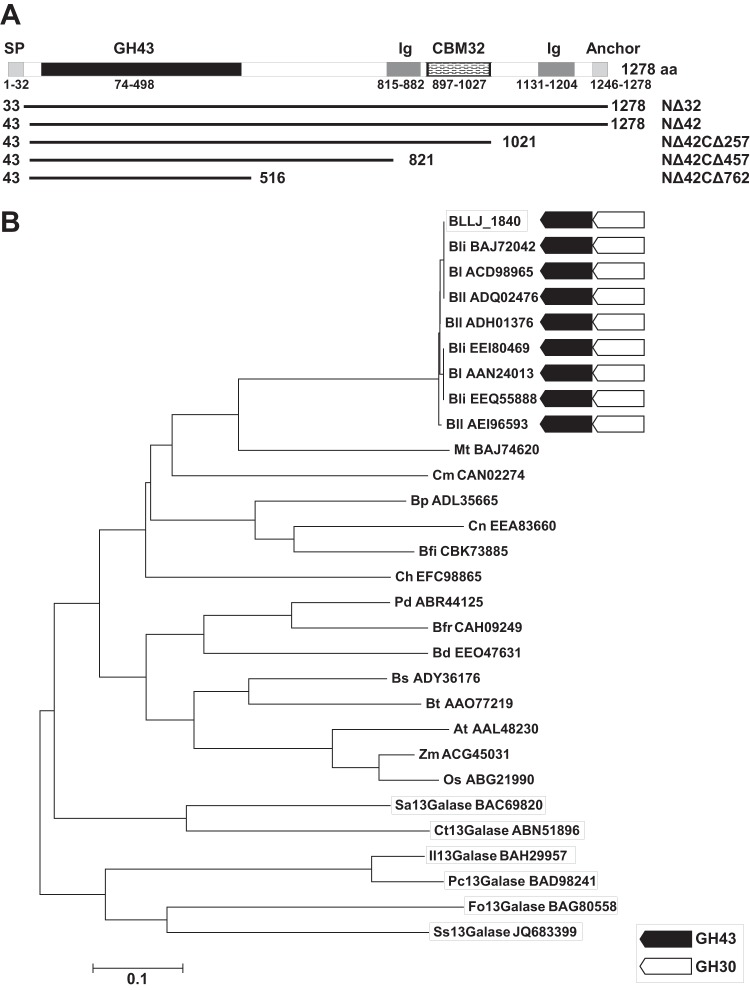
BLLJ_1840 comprises 1,278 aa, including a putative signal peptide, a GH43 catalytic domain, two bacterial Ig-like domains, a carbohydrate-binding module (CBM) 32 (CBM32) domain, and an LPXTG cell wall anchor domain (Fig. 1A). The amino acid sequence of the GH43 catalytic domain had 27% to 28% identity with other characterized exo-β-1,3-galactanases from Clostridium thermocellum (Ct1,3Gal), Irpex lacteus (Il1,3Gal), Streptomyces avermitilis (Sa1,3Gal), a Streptomyces sp. (Ss1,3Gal), Phanerochaete chrysosporium (Pc1,3Gal), and Fusarium oxysporum (Fo1,3Gal) (Fig. 1B). The catalytic residues of exo-β-1,3-galactanase were recently predicted by crystallographic studies of Ct1,3Gal (26); these residues were conserved in BLLJ_1840 (see Fig. S1 in the supplemental material). The predicted catalytic residues for BLLJ_1840 were as follows: Glu196 (a general base), Asp274 (a modulator of the general acid), and Glu359 (a general acid). In addition to B. longum subsp. longum and B. longum subsp. infantis, BLLJ_1840 homologous genes were also conserved in some intestinal bacteria, including Bacteroides spp., Clostridium spp., and Butyrivibrio spp. (Fig. 1B) and in some actinobacteria and plants (Fig. 1B).
FIG 1.
Domain structures and phylogenetic relationships of BLLJ_1840. (A) BLLJ_1840 comprises the GH43 catalytic domain, bacterial Ig-like domain (Ig), CBM32, and the LPXTG cell wall anchor domain (Anchor). The signal peptide (SP) was predicted using the SignalP (version 4.1) server (http://www.cbs.dtu.dk/services/SignalP/). The lines indicate the length of the BLLJ_1840 deletion mutants. (B) The phylogenetic tree of BLLJ_1840 with homologous proteins was constructed with the neighbor-joining method using the aligned sequences with the MUSCLE program implemented in MEGA5 software. BLLJ_1840 and the characterized exo-β-1,3-galactanases are boxed. Black and white arrows, conservation of GH43 exo-β-1,3-galactanases and the neighboring GH30 endo-β-1,6-galactanase candidates, respectively. The GenBank accession numbers are shown with the abbreviations for the organisms, which are as follows: Bli, B. longum subsp. infantis; Bl, B. longum; Bll, B. longum subsp. longum; Mt, Microbacterium testaceum; Cm, Clavibacter michiganensis; Bp, Butyrivibrio proteoclasticus; Cn, Clostridium nexile; Bfi, Butyrivibrio fibrisolvens; Ch, Clostridium hathewayi; Pd, Parabacteroides distasonis; Bfr, Bacteroides fragilis; Bd, Bacteroides dorei; Bs, Bacteroides salanitronis; Bt, Bacteroides thetaiotaomicron; At, Arabidopsis thaliana; Zm, Zea mays; Os, Oryza sativa; Sa, Streptomyces avermitilis; Ct, Clostridium thermocellum; Il, Irpex lacteus; Pc, Phanerochaete chrysosporium; Fo, Fusarium oxysporum; Ss, Streptomyces sp.
Preparation of the recombinant BLLJ_1840 protein.
The recombinant BLLJ_1840-NΔ32 protein, which was designed without the N-terminal signal peptide, was detected at only a low expression level on SDS-polyacrylamide gels (data not shown). To obtain a highly expressed clone, four deletion mutants, BLLJ_1840-NΔ42, -NΔ42CΔ257, -NΔ42CΔ457, and -NΔ42CΔ762, were constructed (Fig. 1A). The BLLJ_1840-NΔ42, -NΔ42CΔ257, and -NΔ42CΔ457 proteins maintained their enzymatic activities, whereas BLLJ_1840-NΔ42CΔ762 showed only 8.45% of the activity of BLLJ_1840-NΔ32 (Table 1). Because BLLJ_1840-NΔ42CΔ257 was highly expressed as a soluble protein at 20°C, we selected it for further analysis. The purified recombinant BLLJ_1840-NΔ42CΔ257 protein migrated as a single band with an apparent molecular mass of 106 kDa in SDS-polyacrylamide gels (see Fig. S2 in the supplemental material) and was found at m/z 105,626 by MALDI-TOF MS (data not shown). These molecular masses were in agreement with the calculated molecular mass of 106,128 Da.
TABLE 1.
Specific activities of BLLJ_1840 deletion mutants
| Deletion mutant | Sp act (units/mg) | Relative activitya (%) |
|---|---|---|
| NΔ42 | 20.2 | 100 |
| NΔ42CΔ257 | 16.2 | 80.1 |
| NΔ42CΔ457 | 24.6 | 122 |
| NΔ42CΔ762 | 1.71 | 8.45 |
Relative activity was expressed as the percentage of the activity toward BLLJ_1840-NΔ42.
Characterization of recombinant BLLJ_1840.
To identify the substrate specificities of BLLJ_1840-NΔ42CΔ257, several galactooligosaccharides and synthetic pNP substrates were used. The enzyme released galactose from β-1,3-Gal2, -Gal3, -galactotetraose (Gal4), and -galactopentaose (Gal5) (Fig. 2). The catalytic efficiency was less affected by the size of the oligosaccharides (Table 2). However, the enzyme did not have any activity toward β-1,4-galactooligosaccharides, such as β-1,4-Gal2, -Gal3, and -Gal4, or toward β-1,6-galactooligosaccharides, such as β-1,6-Gal2, -Gal3, and -Gal4 (data not shown). Furthermore, the enzyme had no degradative activity for pNP substrates, such as pNP–β-d-galactopyranoside, pNP–α-d-galactopyranoside, pNP–β-d-glucopyranoside, pNP–β-d-xylopyranoside, and pNP–β-l-arabinopyranoside (data not shown).
FIG 2.
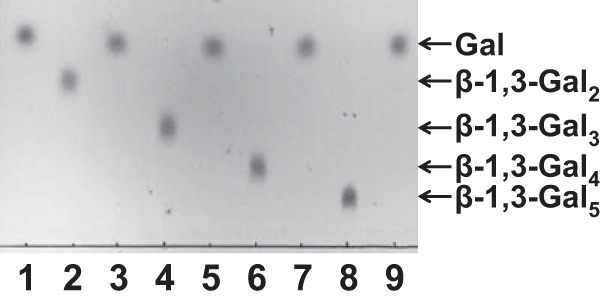
TLC analysis of BLLJ_1840 reactions. β-1,3-Galactooligosaccharides were incubated in the absence (lanes 2, 4, 6, and 8) or presence (lanes 3, 5, 7, and 9) of the recombinant enzyme at 40°C for 16 h. Lane 1, galactose standard.
TABLE 2.
Substrate specificity of BLLJ_1840 toward β-1,3-galactooligosaccharides
| Substratea | Sp act (units/mg) | Relative activityb (%) |
|---|---|---|
| β-1,3-Gal2 | 0.0218 | 100 |
| β-1,3-Gal3 | 0.0188 | 85.9 |
| β-1,3-Gal4 | 0.0139 | 63.7 |
| β-1,3-Gal5 | 0.0157 | 71.5 |
The final concentration of the substrates was 0.1 mM.
Relative activity was expressed as the percentage of the activity toward β-1,3-Gal2.
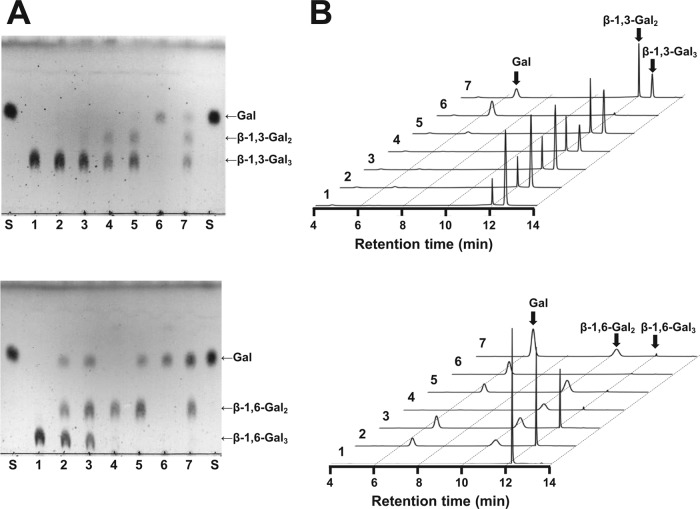
Next, natural and chemically modified polysaccharides were used to identify the substrates of BLLJ_1840. The released oligosaccharides were analyzed by HPAEC-PAD. As shown in Fig. 3, the enzyme released galactose, β-1,6-Gal2, β-1,6-Gal3, β-1,6-Gal4, β-1,6-Gal3 substituted with a single α-l-arabinofuranoside (Araf-Gal3), and β-l-arabinopyranosyl Araf-Gal3 (Arap-Araf-Gal3) from LWAG. β-1,6-Gal4 was a minor component and had the same retention time as β-1,6-Gal3 on HPAEC-PAD under the conditions described in Materials and Methods. The purified Araf-Gal3 was degraded to Araf-Gal2 by A. niger β-galactosidase. Following the hydrolysis by A. niger α-l-arabinofuranosidase, the main construct was β-1,6-Gal2, which suggested that a single α-l-arabinofuranoside was substituted on a middle position of galactose on β-1,6-Gal3. In addition, unidentified oligosaccharides (retention times, 14.5 to 18.0 min) were detected as minor components (Fig. 3). The enzyme also released galactose and β-1,6-galactooligosaccharides from dearabinosylated LWAG, β-1,3-galactan, dearabinosylated gum arabic, and gum arabic. However, the enzyme did not release reaction products from laminarin, a β-1,3/β-1,6-glucan, or potato galactan, a β-1,4-galactan (Table 3). These results showed that BLLJ_1840 is an exo-β-1,3-galactanase with high degradation activity for dearabinosylated AG-II. Next, we investigated the mode of action of BLLJ_1840-NΔ42CΔ257 using dearabinosylated LWAG, LWAG, and β-1,3-galactan as the substrates (Fig. 4). In the early-phase reaction, β-1,6-Gal2 and β-1,6-Gal3 were released from dearabinosylated LWAG, whereas these releases were suppressed on LWAG, suggesting that the substituted l-arabinose inhibited the enzymatic activity. In addition, the enzyme released saccharides from β-1,3-galactan more slowly than those from dearabinosylated LWAG, suggesting that the enzyme has a higher affinity for the β-1,3-galactan substituted with β-1,6-galactan than for nonsubstituted β-1,3-galactan.
FIG 3.
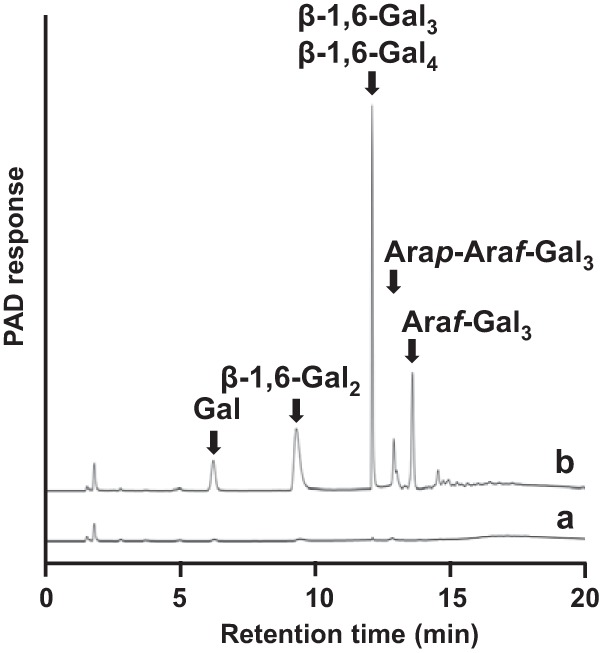
HPAEC-PAD analysis of BLLJ_1840 reactions. LWAG was incubated with (curve a) or without (curve b) BLLJ_1840-NΔ42CΔ257 at 40°C for 16 h.
TABLE 3.
Substrate specificity of BLLJ_1840 toward polysaccharides
| Substratea | Sp act (units/mg) | Relative activityb (%) |
|---|---|---|
| Dearabinosylated LWAG | 16.2 | 100 |
| LWAG | 1.17 | 7.19 |
| β-1,3-Galactan | 1.01 | 6.25 |
| Dearabinosylated gum arabic | 1.28 | 7.90 |
| Gum arabic | 0.113 | 0.699 |
| Laminarin (β-1,3/β-1,6-glucan) | NDc | ND |
| Potato galactan (β-1,4-galactan) | ND | ND |
The final concentration of the substrates was 1.0% (wt/vol).
Relative activity was expressed as the percentage of the activity toward dearabinosylated LWAG.
ND, cleavage of the substrate was not detected.
FIG 4.

Time course of degradation of polysaccharides by BLLJ_1840. Dearabinosylated LWAG (A), LWAG (B), and β-1,3-galactan (C) were incubated with BLLJ_1840-NΔ42CΔ257 at 40°C for up to 320 min. The released saccharides were quantified by HPAEC-PAD. The sugars tested were galactose (open circles), β-1,6-Gal2 (closed circles), β-1,6-Gal3 (open squares), and Araf-Gal3 (closed squares).
The optimal temperature and pH for dearabinosylated LWAG were 40°C and 5.5, respectively (see Fig. S3 in the supplemental material). The Km and kcat values for dearabinosylated LWAG were calculated to be 20.7 mg/ml and 78.1 s−1, respectively (Table 4). In contrast, the kcat value for β-1,3-galactan was 25.4-fold lower than that for dearabinosylated LWAG. The Km and kcat values for LWAG were 6.1-fold higher and 5.2-fold lower than those for dearabinosylated LWAG, respectively. Consequently, kcat/Km values for LWAG and β-1,3-galactan were 31.9-fold and 23.6-fold lower than those for dearabinosylated LWAG, respectively.
TABLE 4.
Kinetic parameters of BLLJ_1840 activity on different substrates
| Substrate | Km (mg/ml) | kcat (s−1) | kcat/Km [ml/(mg · s)] |
|---|---|---|---|
| Dearabinosylated LWAG | 20.7 ± 6.3 | 78.1 ± 20.5 | 3.77 |
| LWAG | 126 ± 22.6 | 14.9 ± 1.9 | 0.118 |
| β-1,3-Galactan | 19.3 ± 4.7 | 3.08 ± 0.35 | 0.160 |
Carbohydrate availability and gene expression profiles of B. longum subsp. longum.
PYF medium containing 1.0% l-arabinose, glucose, galactose, β-1,6-Gal2, β-1,6-Gal3, or LWAG was utilized as the carbohydrate source by B. longum subsp. longum JCM1217 (see Fig. S4 in the supplemental material). In particular, LWAG and glucose were suitable for the growth of B. longum subsp. longum. The expression profiles of BLLJ_1840 and BLLJ_1841 were examined in B. longum subsp. longum grown in the carbohydrate sources (Fig. 5). In comparison with glucose, the transcript levels of BLLJ_1840 and BLLJ_1841 increased 109-fold and 32-fold on LWAG, respectively. These genes were also induced 1.8- to 19-fold by the LWAG degradative sugars, such as l-arabinose, galactose, β-1,6-Gal2, and β-1,6-Gal3. These data suggest that BLLJ_1840 and BLLJ_1841 were simultaneously produced by the synergistic effect of AG-II degradative sugars. In addition, β-1,6-Gal3 was partly degraded by B. longum subsp. longum cultivated without a carbohydrate source (Fig. 6). However, degradative activities for β-1,3-Gal3 were found in B. longum subsp. longum grown in galactose, gum arabic, LWAG, and extensin, but not in B. longum subsp. longum grown in l-arabinose or a sugar-free environment. In particular, B. longum subsp. longum grown in LWAG completely degraded β-1,3-Gal3 and β-1,6-Gal3. These data are consistent with those from transcriptional analysis.
FIG 5.
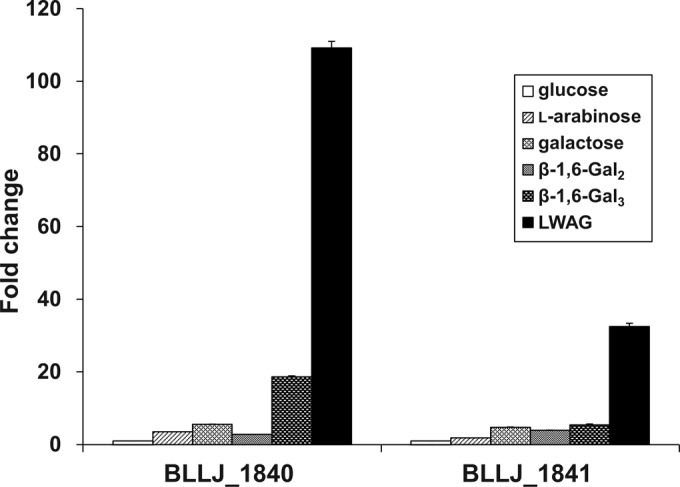
Transcript levels of BLLJ_1840 and BLLJ_1841 in B. longum subsp. longum grown on several carbohydrate sources. The fold changes were expressed from the values measured in mRNA from B. longum subsp. longum grown on glucose. Data are indicated as the mean ± SEM (n = 3).
FIG 6.
Detection of the degradative activities toward AG-II in B. longum subsp. longum. The results of TLC (A) and HPAEC-PAD (B) analyses of the reaction products are shown. The cell wall fractions of B. longum subsp. longum JCM1217 grown in PYF medium containing 1.0% l-arabinose (lanes 3), galactose (lanes 4), gum arabic (lanes 5), LWAG (lanes 6), or extensin (lanes 7) were incubated with β-1,3-Gal3 (top) and β-1,6-Gal3 (bottom) at 40°C for 16 h. Lanes 1, control without B. longum subsp. longum cell wall fraction; lanes 2, B. longum subsp. longum cells grown in PYF medium without carbohydrate source; lanes S, galactose standard. The curve numbers in panel B correspond the lane numbers in panel A.
DISCUSSION
BLLJ_1840 showed higher activity with dearabinosylated LWAG than with β-1,3-galactan. To date, all characterized exo-β-1,3-galactanases have greater activity toward β-1,3-galactan than β-1,3/β-1,6-galactan from Prototheca zopfii, LWAG, gum arabic, and radish roots (1, 3–7, 21). BLLJ_1840 has low sequence identities (27% to 28%) with other exo-β-1,3-galactanases. Furthermore, in the patterns from multiple-sequence alignment, there are several gap sequences in the zones corresponding to GH43 catalytic regions from exo-β-1,3-galactanases (see Fig. S1 in the supplemental material). Thus, the active-site structure of BLLJ_1840 may be suitable for the β-1,6-branched substrates. In contrast, BLLJ_1840 showed 7.90% of the enzymatic activity for dearabinosylated gum arabic compared with the corresponding activity for dearabinosylated LWAG (Table 3). Gum arabic has highly branched side chains with uronic acid and l-rhamnose residues, in addition to l-arabinose (27). Therefore, additional enzymatic treatments using β-galacturonidase, β-glucuronidase, and α-l-rhamnosidase may be needed for the degradation of gum arabic.
All the characterized exo-β-1,3-galactanases contain a C-terminal CBM domain. Three fungal enzymes (Il1,3Gal, Pc1,3Gal, and Fo1,3Gal) have CBM35, and three bacterial enzymes (Ct1,3Gal, Sa1,3Gal, and Ss1,3Gal) have CBM13. BLLJ_1840 is the first characterized exo-β-1,3-galactanase containing a C-terminal CBM32 domain. The CBM32 domain is found in other bifidobacterial glycosidases, such as GH101 endo-α-N-acetylgalactosaminidase (17), GH121 β-l-arabinobiosidase (18), GH29 α-l-fucosidase (28), and GH20 lacto-N-biosidase (29). The bacterial Ig-like domain is also found in bifidobacterial glycosidases, such as GH20 lacto-N-biosidase and GH95 α-l-fucosidase (30). A C-terminal LPXTG membrane-anchoring motif is found in several surface proteins from Gram-positive bacteria. These C-terminal domains are conserved in all the homologous genes from bifidobacteria shown in Fig. 1B. CBMs in bifidobacteria are predicted to be docking stations for substrates and/or hydrolysates (31). The CBM32 domain is considered to bind galactose or its derivatives (32). These C-terminal domains may act as docking stations for AG-II and/or the hydrolysates on the bifidobacterial cell surface for the acquisition of sugar nutrients.
BLLJ_1840 forms a gene cluster with a GH30 endo-β-1,6-galactanase (BLLJ_1841) which hydrolyzes the side chains of AG-II. These enzymes are predicted to localize on the bifidobacterial cell surface. These homologous genes are conserved in many strains of B. longum subsp. longum and B. longum subsp. infantis but not in other bifidobacteria (Fig. 1B). In addition, the gene cluster is flanked by one homologous gene of β-l-arabinofuranosidase (BLLJ_1848) and five duplicated genes of GH43 α-l-arabinofuranosidases (BLLJ_1850 to BLLJ_1854) in B. longum subsp. longum JCM1217. In the presence of AG-II, the transcript levels of BLLJ_1840 and BLLJ_1841 increased, as did their AG-II degradative activity, in B. longum subsp. longum. In addition, microarray analysis has shown that genes homologous to BLLJ_1840 and BLLJ_1841 were induced in B. longum subsp. longum by commercial β-galactooligosaccharides, which contain a mixture of β-1,4- and β-1,6-galactosyl linkages (33). Therefore, we predict that the presence of exo-β-1,3-galactanase and endo-β-1,6-galactanase is the reason why B. longum subsp. longum can assimilate AG-II.
B. longum subsp. longum JCM1217 encodes three homologous genes of intracellular β-galactosidases; BLLJ_1486 belongs to GH2, and BLLJ_0352 and BLLJ_0443 belong to GH42. Blon_2016 from B. longum subsp. infantis, which has 96% identity with BLLJ_0443, showed a higher specificity for β-1,3-Gal2 and β-1,6-Gal2 than for β-1,4-Gal2 and lactose (34). Conversely, Blon_2334 (59% identity with BLLJ_1486) had high specificities for lactose and N-acetyllactosamine (35), and Blon_2123 (71% identity with BLLJ_0352) had specificity for β-1,4-Gal2 (34). In addition, B. longum subsp. longum had increased intracellular β-galactosidase activity in the presence of gum arabic (16), and an inducible β-galactosidase was identified as a BLLJ_0443 homologue (36). Thus, BLLJ_0443 may have a key role in the degradation of β-1,6-Gal2 in B. longum subsp. longum. The neighboring BLLJ_0445 encodes an intracellular GH51 α-l-arabinofuranosidase. AbfB from B. longum subsp. longum B667 (100% identity with BLLJ_0445) had broad substrate specificity for pNP–α-l-arabinofuranoside, arabinan, and arabinoxylan (37). Furthermore, the gene cluster contains three candidates of solute-binding proteins (BLLJ_0446 to BLLJ_0448) and two permeases (BLLJ_0441 and BLLJ_0442), which were predicted to be an ABC transport system for lactose and fructooligosaccharides in B. longum NCC2705 (38). We also confirmed the transcriptional induction of the BLLJ_0446 to BLLJ_0448 genes by LWAG (unpublished data). Therefore, the gene cluster (BLLJ_0441 to BLLJ_0448) may encode ABC transport systems and the intracellular degradative enzymes for AG-II metabolism.
The AG-II metabolic pathway in B. longum subsp. longum was predicted and is shown in Fig. S5 in the supplemental material. First, AG-II is degraded by exo-β-1,3-galactanase (BLLJ_1840), endo-β-1,6-galactanase (BLLJ_1841), and GH43 α-l-arabinofuranosidases (BLLJ_1850 to BLLJ_1854) on the bifidobacterial cell surface. Next, the released l-arabinose, galactose, β-1,6-Gal2, and undegraded β-1,6-galactooligosaccharides containing Araf-Gal3 are internalized into the bifidobacterial cell by ABC transport systems, as described above. Then, β-1,6-Gal2 and Araf-Gal3 are degraded to galactose and l-arabinose by the intracellular α-l-arabinofuranosidase (BLLJ_0445) and β-galactosidase (BLLJ_0443). Finally, the released galactose and l-arabinose are metabolized by the Leloir pathway (39) and the l-arabinose metabolic pathway (40), respectively. We recently proposed a metabolic pathway for the β-l-arabinooligosaccharide chain of extensin based on the characterization of the enzymes encoded in a gene cluster (BLLJ_0207 to BLLJ_0213) of B. longum subsp. longum (40). Extensin and AGP are common members of hydroxyproline-rich glycoprotein (HRGP) in plants. The gum arabic glycoprotein contains both modules of extensin and AGP (41). Therefore, both metabolic pathways for AG-II and β-l-arabinooligosaccharides are considered essential for gum arabic fermentation in B. longum subsp. longum.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by a JSPS KAKENHI Grant-in-Aid for Scientific Research (C), grant number 24580144.
Footnotes
Published ahead of print 16 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00802-14.
REFERENCES
- 1.Ichinose H, Yoshida M, Kotake T, Kuno A, Igarashi K, Tsumuraya Y, Samejima M, Hirabayashi J, Kobayashi H, Kaneko S. 2005. An exo-β-1,3-galactanase having a novel β-1,3-galactan-binding module from Phanerochaete chrysosporium. J. Biol. Chem. 280:25820–25829. 10.1074/jbc.M501024200 [DOI] [PubMed] [Google Scholar]
- 2.Tsumuraya Y, Mochizuki N, Hashimoto Y, Kovac P. 1990. Purification of an exo-β-(1→3)-d-galactanase of Irpex lacteus (Polyporus tulipiferae) and its action on arabinogalactan-proteins. J. Biol. Chem. 265:7207–7215 [PubMed] [Google Scholar]
- 3.Okawa M, Fukamachi K, Tanaka H, Sakamoto T. 2013. Identification of an exo-β-1,3-d-galactanase from Fusarium oxysporum and the synergistic effect with related enzymes on degradation of type II arabinogalactan. Appl. Microbiol. Biotechnol. 97:9685–9694. 10.1007/s00253-013-4759-3 [DOI] [PubMed] [Google Scholar]
- 4.Ling NX, Lee J, Ellis M, Liao ML, Mau SL, Guest D, Janssen PH, Kovac P, Bacic A, Pettolino FA. 2012. An exo-β-(1→3)-d-galactanase from Streptomyces sp. provides insights into type II arabinogalactan structure. Carbohydr. Res. 352:70–81. 10.1016/j.carres.2012.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotake T, Kitazawa K, Takata R, Okabe K, Ichinose H, Kaneko S, Tsumuraya Y. 2009. Molecular cloning and expression in Pichia pastoris of a Irpex lacteus exo-β-(1→3)-galactanase gene. Biosci. Biotechnol. Biochem. 73:2303–2309. 10.1271/bbb.90433 [DOI] [PubMed] [Google Scholar]
- 6.Ichinose H, Kuno A, Kotake T, Yoshida M, Sakka K, Hirabayashi J, Tsumuraya Y, Kaneko S. 2006. Characterization of an exo-β-1,3-galactanase from Clostridium thermocellum. Appl. Environ. Microbiol. 72:3515–3523. 10.1128/AEM.72.5.3515-3523.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichinose H, Kotake T, Tsumuraya Y, Kaneko S. 2006. Characterization of an exo-β-1,3-d-galactanase from Streptomyces avermitilis NBRC14893 acting on arabinogalactan-proteins. Biosci. Biotechnol. Biochem. 70:2745–2750. 10.1271/bbb.60365 [DOI] [PubMed] [Google Scholar]
- 8.Cassab GI. 1986. Arabinogalactan proteins during the development of soybean root nodules. Planta 168:441–446. 10.1007/BF00392262 [DOI] [PubMed] [Google Scholar]
- 9.Capek P, Matulová M, Navarini L, Suggi-Liverani F. 2010. Structural features of an arabinogalactan-protein isolated from instant coffee powder of Coffea arabica beans. Carbohydr. Polym. 80:180–185. 10.1016/j.carbpol.2009.11.016 [DOI] [Google Scholar]
- 10.Göllner EM, Blaschek W, Classen B. 2010. Structural investigations on arabinogalactan-protein from wheat, isolated with Yariv reagent. J. Agric. Food Chem. 58:3621–3626. 10.1021/jf903843f [DOI] [PubMed] [Google Scholar]
- 11.Guadalupe Z, Martínez-Pinilla O, Garrido Á, Carrillo JD, Ayestarán B. 2012. Quantitative determination of wine polysaccharides by gas chromatography–mass spectrometry (GC–MS) and size exclusion chromatography (SEC). Food Chem. 131:367–374. 10.1016/j.foodchem.2011.08.049 [DOI] [Google Scholar]
- 12.Tsumuraya Y, Ogura K, Hashimoto Y, Mukoyama H, Yamamoto S. 1988. Arabinogalactan-proteins from primary and mature roots of radish (Raphanus sativus L.). Plant Physiol. 86:155–160. 10.1104/pp.86.1.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calame W, Weseler AR, Viebke C, Flynn C, Siemensma AD. 2008. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br. J. Nutr. 100:1269–1275. 10.1017/S0007114508981447 [DOI] [PubMed] [Google Scholar]
- 14.Cherbut C, Michel C, Raison V, Kravtchenko T, Severine M. 2003. Acacia gum is a bifidogenic dietary fiber with high digestive tolerance in healthy humans. Microb. Ecol. Health Dis. 15:43–50. 10.1080/08910600310014377 [DOI] [Google Scholar]
- 15.Crociani F, Alessandrini A, Mucci MM, Biavati B. 1994. Degradation of complex carbohydrates by Bifidobacterium spp. Int. J. Food Microbiol. 24:199–210. 10.1016/0168-1605(94)90119-8 [DOI] [PubMed] [Google Scholar]
- 16.Degnan BA, Macfarlane GT. 1995. Arabinogalactan utilization in continuous cultures of Bifidobacterium longum: effect of co-culture with Bacteroides thetaiotaomicron. Anaerobe 1:103–112. 10.1006/anae.1995.1005 [DOI] [PubMed] [Google Scholar]
- 17.Fujita K, Oura F, Nagamine N, Katayama T, Hiratake J, Sakata K, Kumagai H, Yamamoto K. 2005. Identification and molecular cloning of a novel glycoside hydrolase family of core 1 type O-glycan-specific endo-α-N-acetylgalactosaminidase from Bifidobacterium longum. J. Biol. Chem. 280:37415–37422. 10.1074/jbc.M506874200 [DOI] [PubMed] [Google Scholar]
- 18.Fujita K, Sakamoto S, Ono Y, Wakao M, Suda Y, Kitahara K, Suganuma T. 2011. Molecular cloning and characterization of a β-l-arabinobiosidase in Bifidobacterium longum that belongs to a novel glycoside hydrolase family. J. Biol. Chem. 286:5143–5150. 10.1074/jbc.M110.190512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling NX-Y, Pettolino F, Liao M-L, Bacic A. 2009. Preparation of a new chromogenic substrate to assay for β-galactanases that hydrolyse type II arabino-3,6-galactans. Carbohydr. Res. 344:1941–1946. 10.1016/j.carres.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 20.Tsumuraya Y, Hashimoto Y, Yamamoto S, Shibuya N. 1984. Structure of l-arabino-d-galactan-containing glycoproteins from radish leaves. Carbohydr. Res. 134:215–228. 10.1016/0008-6215(84)85039-9 [DOI] [Google Scholar]
- 21.Sakamoto T, Tanaka H, Nishimura Y, Ishimaru M, Kasai N. 2011. Characterization of an exo-β-1,3-d-galactanase from Sphingomonas sp. 24T and its application to structural analysis of larch wood arabinogalactan. Appl. Environ. Biotechnol. 90:1701–1710. 10.1007/s00253-011-3219-1 [DOI] [PubMed] [Google Scholar]
- 22.Smogyi M. 1952. Notes on sugar determination. J. Biol. Chem. 195:19–23 [PubMed] [Google Scholar]
- 23.Holmes EW, O'Brien JS. 1979. Separation of glycoprotein-derived oligosaccharides by thin-layer chromatography. Anal. Biochem. 93:167–170. 10.1016/S0003-2697(79)80131-1 [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto M, Yamakawa O, Tanoue H. 2005. Potential chemopreventive properties and varietal difference of dietary fiber from sweetpotato (Ipomoea batatas L.) root. Jpn. Agric. Res. Q. 39:37–43. 10.6090/jarq.39.37 [DOI] [Google Scholar]
- 25.Savard P, Roy D. 2009. Determination of differentially expressed genes involved in arabinoxylan degradation by Bifidobacterium longum NCC2705 using real-time RT-PCR. Probiotics Antimicrob. Proteins 1:121–129. 10.1007/s12602-009-9015-x [DOI] [PubMed] [Google Scholar]
- 26.Jiang D, Fan J, Wang X, Zhao Y, Huang B, Liu J, Zhang XC. 2012. Crystal structure of 1,3Gal43A, an exo-β-1,3-galactanase from Clostridium thermocellum. J. Struct. Biol. 180:447–457. 10.1016/j.jsb.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 27.Nie S-P, Wang C, Cui SW, Wang Q, Xie M-Y, Phillips GO. 2013. A further amendment to the classical core structure of gum arabic (Acacia senegal). Food Hydrocolloids 31:42–48. 10.1016/j.foodhyd.2012.09.014 [DOI] [Google Scholar]
- 28.Ashida H, Miyake A, Kiyohara M, Wada J, Yoshida E, Kumagai H, Katayama T, Yamamoto K. 2009. Two distinct α-l-fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates. Glycobiology 19:1010–1017. 10.1093/glycob/cwp082 [DOI] [PubMed] [Google Scholar]
- 29.Wada J, Ando T, Kiyohara M, Ashida H, Kitaoka M, Yamaguchi M, Kumagai H, Katayama T, Yamamoto K. 2008. Bifidobacterium bifidum lacto-N-biosidase, a critical enzyme for the degradation of human milk oligosaccharides with a type 1 structure. Appl. Environ. Microbiol. 74:3996–4004. 10.1128/AEM.00149-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katayama T, Sakuma A, Kimura T, Makimura Y, Hiratake J, Sakata K, Yamanoi T, Kumagai H, Yamamoto K. 2004. Molecular cloning and characterization of Bifidobacterium bifidum 1,2-α-l-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J. Bacteriol. 186:4885–4893. 10.1128/JB.186.15.4885-4893.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Broek LA, Hinz SW, Beldman G, Vincken JP, Voragen AG. 2008. Bifidobacterium carbohydrases—their role in breakdown and synthesis of (potential) prebiotics. Mol. Nutr. Food Res. 52:146–163. 10.1002/mnfr.200700121 [DOI] [PubMed] [Google Scholar]
- 32.Abbott DW, Eirin-Lopez JM, Boraston AB. 2008. Insight into ligand diversity and novel biological roles for family 32 carbohydrate-binding modules. Mol. Biol. Evol. 25:155–167. 10.1093/molbev/msm243 [DOI] [PubMed] [Google Scholar]
- 33.González R, Klaassens ES, Malinen E, de Vos WM, Vaughan EE. 2008. Differential transcriptional response of Bifidobacterium longum to human milk, formula milk, and galactooligosaccharide. Appl. Environ. Microbiol. 74:4686–4694. 10.1128/AEM.00122-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viborg AH, Katayama T, Hachem MA, Andersen MC, Nishimoto M, Clausen MH, Urashima T, Svensson B, Kitaoka M. 2014. Distinct substrate specificities of three glycoside hydrolase family 42 β-galactosidases from Bifidobacterium longum subsp. infantis ATCC 15697. Glycobiology 24:208–216. 10.1093/glycob/cwt104 [DOI] [PubMed] [Google Scholar]
- 35.Yoshida E, Sakurama H, Kiyohara M, Nakajima M, Kitaoka M, Ashida H, Hirose J, Katayama T, Yamamoto K, Kumagai H. 2012. Bifidobacterium longum subsp. infantis uses two different β-galactosidases for selectively degrading type-1 and type-2 human milk oligosaccharides. Glycobiology 22:361–368. 10.1093/glycob/cwr116 [DOI] [PubMed] [Google Scholar]
- 36.Saishin N, Ueta M, Wada A, Yamamoto I. 2010. Properties of β-galactosidase purified from Bifidobacterium longum subsp. longum JCM 7052 grown on gum arabic. J. Biol. Macromol. 10:23–31 [Google Scholar]
- 37.Margolles A, de los Reyes-Gavilan CG. 2003. Purification and functional characterization of a novel α-l-arabinofuranosidase from Bifidobacterium longum B667. Appl. Environ. Microbiol. 69:5096–5103. 10.1128/AEM.69.9.5096-5103.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parche S, Amon J, Jankovic I, Rezzonico E, Beleut M, Barutçu H, Schendel I, Eddy MP, Burkovski A, Arigoni F, Titgemeyer F. 2007. Sugar transport systems of Bifidobacterium longum NCC2705. J. Mol. Microbiol. Biotechnol. 12:9–19. 10.1159/000096455 [DOI] [PubMed] [Google Scholar]
- 39.Nishimoto M, Kitaoka M. 2007. Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-biose I/galacto-N-biose metabolic pathway in Bifidobacterium longum. Appl. Environ. Microbiol. 73:6444–6449. 10.1128/AEM.01425-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita K, Takashi Y, Obuchi E, Kitahara K, Suganuma T. 2014. Characterization of a novel β-l-arabinofuranosidase in Bifidobacterium longum: functional elucidation of a DUF1680 protein family member. J. Biol. Chem. 289:5240–5249. 10.1074/jbc.M113.528711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodrum LJ, Patel A, Leykam JF, Kieliszewski MJ. 2000. Gum arabic glycoprotein contains glycomodules of both extensin and arabinogalactan-glycoproteins. Phytochemistry 54:99–106. 10.1016/S0031-9422(00)00043-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.