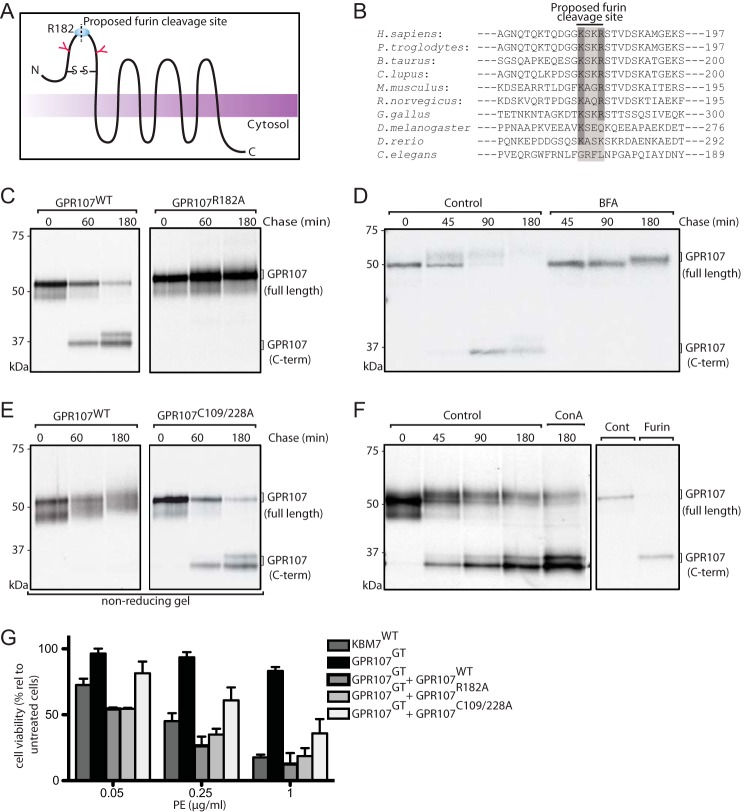
FIGURE 5.
GPR107 is cleaved by furin and the disulfide bond that associates the cleaved fragments is essential for its activity. A, predicted topological structure of GPR107. N-Glycosylation sites, disulfide bond linkage, and the furin cleavage site are indicated. B, sequence alignment of the furin-like cleavage site present at the N-terminal region of GPR107 from different eukaryotes. C, HeLa cells expressing C-terminally HA-tagged GPR107WT and GPR107R182A were labeled with [35S]methionine/cysteine for 30 min and chased for the indicated time points. Cells were lysed and immunoprecipitated with anti-HA coupled beads, subjected to PNGase F treatment, analyzed by SDS-PAGE, and autoradiography. D, BFA untreated and treated C-terminally HA-tagged GPR107WT-expressing HeLa cells were labeled with [35S]methionine/cysteine for 30 min and chased for different time points. Cells were lysed and immunoprecipitated with anti-HA coupled beads, subjected to Endo H treatment, and analyzed as described in C. E, HeLa cells expressing C-terminally HA-tagged GPR107WT and GPR107C109/228A were labeled with [35S]methionine/cysteine for 30 min, chased, and immunoprecipitated as described in C. Unless otherwise indicated, immunoprecipitates were eluted and analyzed in reducing conditions. F, HeLa cells expressing C-terminally HA-tagged GPR107WT were labeled with [35S]methionine/cysteine for 30 min and chased in the presence or absence of the lysosomal inhibitor concanamycin A. Cells were lysed and immunoprecipitated with anti-HA coupled beads, and samples were processed as described in C (left panel). Alternatively, immunoprecipitate at the 0-h chase time point was subjected to furin digestion and analyzed by SDS-PAGE and autoradiography (right panel). G, KBM7WT, GPR107GT, and GPR107GT cells reconstituted with GPR107WT, GPR107R182A, or GPR107C109A/C228A were intoxicated with PE; cell viability was determined and presented as percent relative to nonintoxicated cells. Error bars represent S.D. of three experiments performed in duplicate.