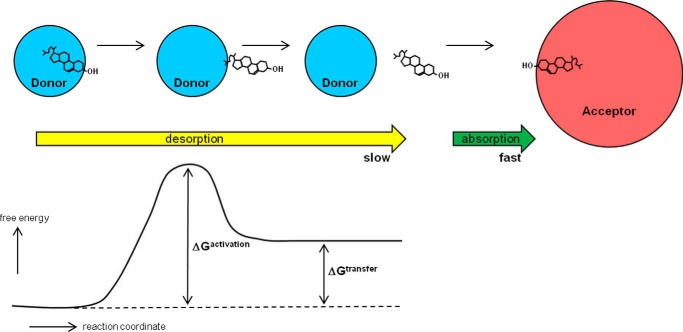
FIGURE 1.
Summary of steps involved in the exchange of cholesterol molecules between PL-containing donor and acceptor particles by the aqueous diffusion mechanism. The rate of transfer of the highly hydrophobic cholesterol molecule from donor to acceptor by this simple diffusion process is limited by the rate of desorption into the aqueous phase. As shown at the left of the diagram, the transition (activated) state involves an almost completely desorbed cholesterol molecule; the free energy of such a molecule that is attached to the donor particle surface by its nonpolar end but has most of its hydrophobic surface exposed to water is high (see the free energy profile). This state is achieved by oscillatory motions of the cholesterol molecule in the plane perpendicular to the surface of the particle. Most of the time, the free energy of a cholesterol molecule in this transition state is reduced by relaxation of the molecule back into the donor particle where the cholesterol molecule is fully solvated by PL acyl chains. Occasionally, a cholesterol molecule desorbs completely into the aqueous phase (net free energy change, ΔGtransfer) where, because of its small size, it diffuses relatively quickly until a collision with an acceptor particle leads to rapid absorption and capture. The flux of cholesterol mass out of the donor particle is given by the product (rate constant for desorption, koff) × (mass of cholesterol in the donor particle). Cholesterol molecules can diffuse in both directions between donor and acceptor particles with the direction of net mass transfer being determined by the concentration (activity) gradient (which approximates to the difference in the cholesterol/PL ratios of the two particles). The physical state of the PL in the particle surface influences the activity (fugacity) of the cholesterol molecules so that koff is dependent on parameters such as degree of PL acyl chain unsaturation and the content of sphingomyelin. See under “Aqueous Diffusion Efflux Pathway” for further details.