FIGURE 1.
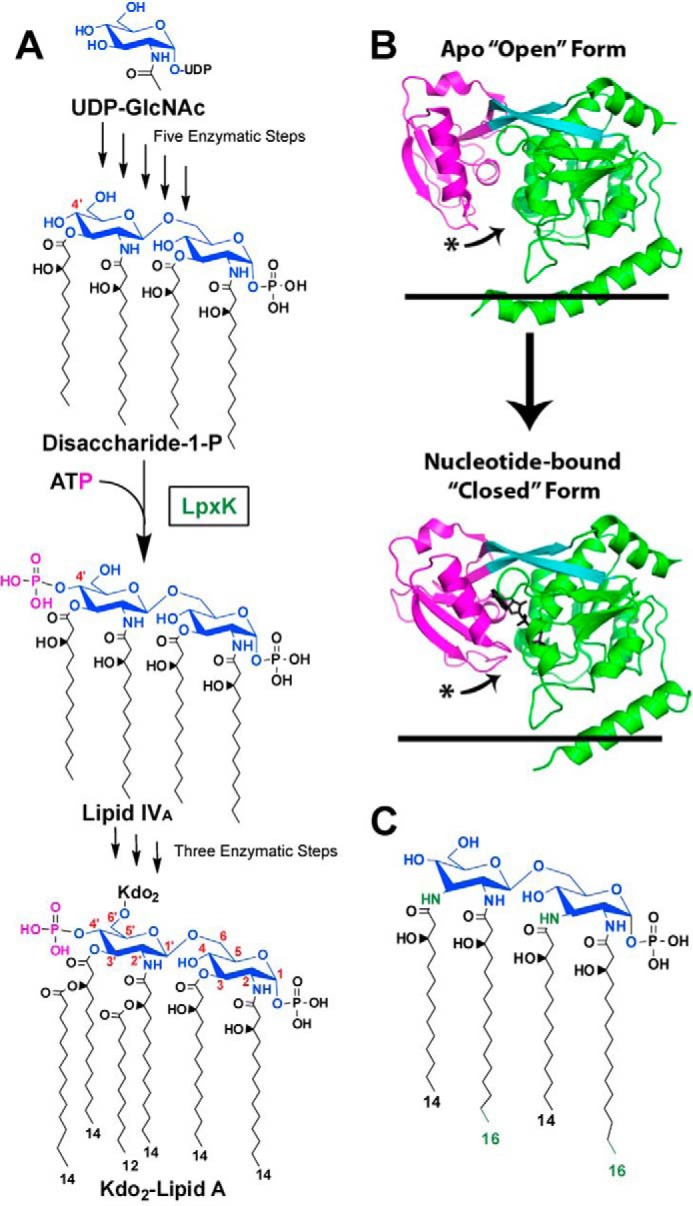
Role of LpxK in the Raetz pathway of lipid A biosynthesis. A, the biosynthesis of lipid A begins with uridine diphosphate-N-acetyl-d-glucosamine (UDP-GlcNAc). The pathway consists of nine steps, the sixth being the phosphorylation (magenta) of DSMP at the 4′-hydroxyl by LpxK, a membrane-bound kinase. Atoms of the glucosamine headgroups of lipid A are highlighted (blue), and positions on these sugars are indicated (red). Acyl chain length in Kdo2-lipid A is also indicated. Kdo, 3-deoxy-d-manno-oct-2-ulosonic acid. B, LpxK consists of an N-terminal domain (green), a linker region (cyan), and a C-terminal domain (magenta). The kinase undergoes a conformational transition upon catalysis in which its C-terminal “lid” domain closes around its nucleotide ligand (black sticks). The curved arrows indicate relative movement of C-terminal loops undergoing this hinge motion. Black lines represent the cytoplasmic inner membrane surface (Protein Data Bank entries 4EHX and 4ITL). C, predicted structure of A. aeolicus DSMP based on the structure of Aquifex lipid A (11, 12). A. aeolicus DSMP contains longer acyl chains at the 2- and 2′-positions on the glucosamine backbone as well as amide linkages for the 3- and 3′-acyl chains, which differentiate it from the E. coli DSMP. Differences are colored in green.
