FIGURE 5.
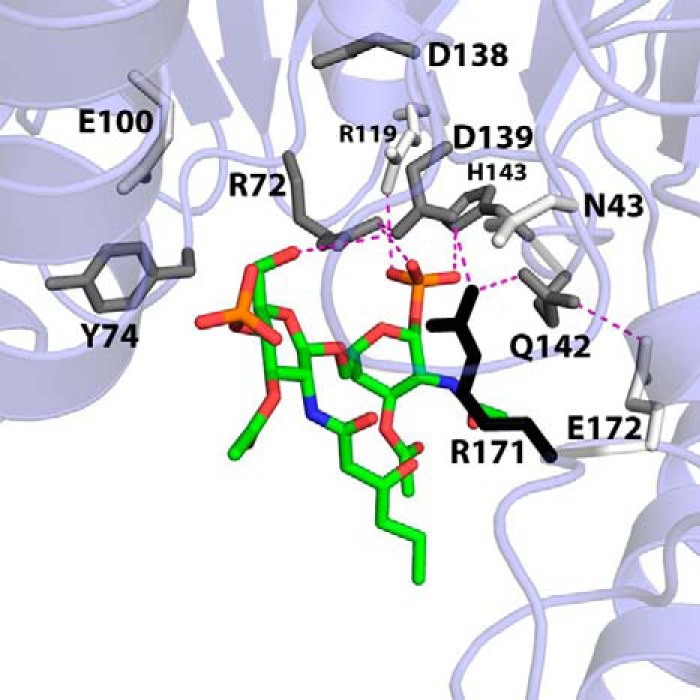
Apparent DSMP Km perturbations of lipid-binding pocket alanine mutants mapped onto the LpxK-lipid IVA coordinates. In order to visualize the effects of alanine mutants of conserved residues within the lipid-binding pocket on the apparent DSMP Km, these residues are shaded in the model based on how much this parameter is increased when mutated to alanine using the following criteria: 0–4-fold Km increase, white; 4–8-fold Km increase, gray; >8-fold Km increase, black. With the exception of Arg119, the alanine mutants of residues that appear to contact the lipid increase the Km at least 4-fold. Arg171 appears to be the most critical residue for lipid substrate binding, with an increased DSMP Km 20-fold higher than wild type. Potential hydrogen bonding interactions are shown as magenta dashes. Font size is indicative of depth.
