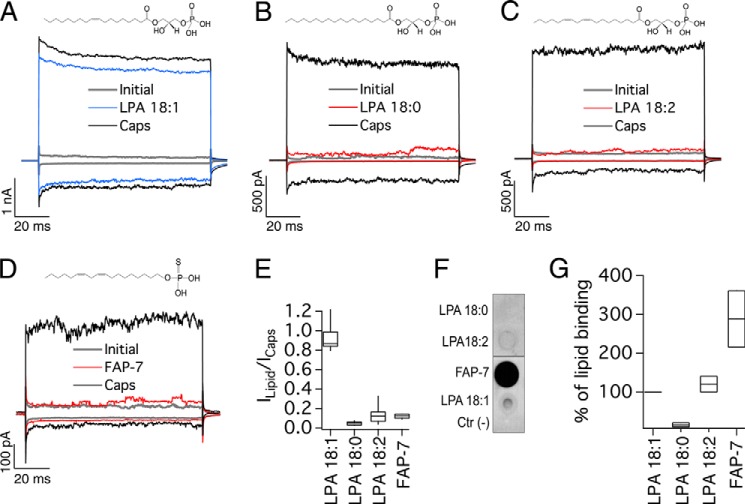
FIGURE 1.
Effects of saturated and unsaturated long-chain analogs of LPA on TRPV1 activation. All representative traces were obtained at −120 and +120 mV. A, initially (leak, gray), after 4 μm capsaicin (Caps, black), and after a wash of capsaicin and with 5 μm LPA 18:1 for 5 min (blue). Currents were obtained from inside-out membrane patches expressing TRPV1. B, initially (in the absence of agonist, gray), after 5 min of 5 μm LPA 18:0 (red), and after 4 μm capsaicin (Caps, black). C, traces obtained initially (gray), after 5 μm LPA 18:2 (red), and after 4 μm capsaicin (Caps, black). D, initial currents are in gray, after 5 min of 5 μm FAP-7 compound (red), and after 4 μm capsaicin (Caps, black). E, box-plot of the activation of TRPV1 by the different lipids. The horizontal line within each box indicates the median; boxes show the 25th and 75th percentiles, and whiskers show the 5th and 95th percentiles of the data obtained at +120 mV and normalized to activation by 4 μm capsaicin (n = 13 for LPA 18:1, 6 for LPA 18:0, 20 for LPA 18:2 and 5 for FAP-7). F, overlay assay with WT TRPV1 protein to show the interaction of the channel with long-chain lipids LPA 18:2 and compound FAP-7. No signal is observed for LPA 18:0, whereas an increase in binding to the channel is observed with LPA 18:2 and compound FAP-7. DMEM with 1% fatty acid-free BSA was used as a negative control indicated as Ctr (−). G, densitometry was performed for the different lipid spots and normalized with respect to the positive control (LPA 18:1). Data are shown as in E (n = 2).