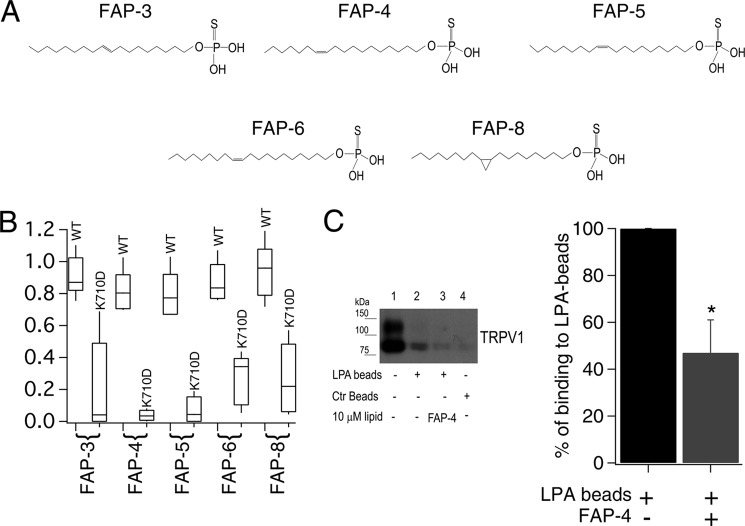
FIGURE 3.
Synthetic long-acyl chain monounsaturated lipids activate TRPV1. A, monounsaturated thiophosphates FAP-3, FAP-4, FAP-5, FAP-6, and the cyclopropyl thiophosphate FAP-8. B, box-plot of the activation of WT TRPV1 and K710D mutant channels by the different lipids. The horizontal line within each box indicates the median; boxes show the 25th and 75th percentiles, and whiskers show the 5th and 95th percentiles of the data obtained at +120 mV and normalized to activation by 4 μm capsaicin (n = 5 and 6 for FAP-4, 6 and 5 for FAP-5, 5 and 5 for FAP-6, 16 and 15 for FAP-3, 5 and 5 for FAP-8, respectively, for WT and TRPV1-K710D). C, (left panel) pulldown assay on plasma membrane protein using LPA-coated beads from HEK293 cells transiently expressing TRPV1. Lane 1 is protein input; lane 2 is TRPV1 pulled down with LPA-coated beads; lane 3 is competition with the FAP-4 lipid; and lane 4 is pulled down protein with control beads (non-LPA-coated beads). Bar chart (right panel) for the average decrease in percentage of binding to LPA beads (obtained after analyzing the intensity of bands as the ones shown in the left panel) before (black) and after (gray) competition in the presence of FAP-4 is shown. Data were normalized to the intensity of the bands obtained only with LPA-coated beads. n = 3; *, p < 0.02 (Student's t test).