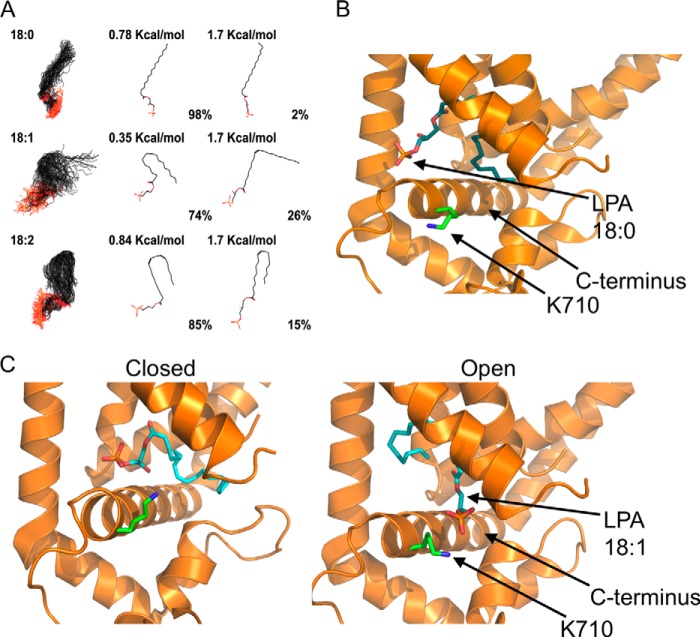
FIGURE 8.
Proposed mechanism for activation of TRPV1 by long-chain lipids. A, LPA 18:0, 18:1, and 18:2 were modeled to simulate an aqueous environment using a Monte Carlo conformational analysis. 1st panel shows all the conformations that may be adopted by the different lipids; 2nd panel shows the conformation adopted under minimal energy requirements; and 3rd panel shows the conformation adopted under a chosen high energy condition. The percentage of molecules found in a given conformation under a certain energetic condition are indicated below the molecules in the 2nd and 3rd panels. B, docking of LPA 18:0 in the minimal energy conformation shown in 2nd panel (0.78 kcal/mol) to the published cryo-EM structure of TRPV1. In this proposed model, there is a lack of interaction of the negatively charged phosphate group of LPA 18:0 with the Lys-710 residue (green) in the proximal C terminus of TRPV1. The structure of LPA 18:0 (cyan) is shown docked to the open state of the channel. C, docking of LPA 18:1 in the minimal energy conformation shown in 2nd panel (0.35 kcal/mol) to the published cryo-EM structure of TRPV1. During opening of the channel, the proximal C terminus helix of TRPV1 could move parallel to the plasma membrane (compare panels on the left for closed state (PDB code 3J5P-B) and on the right for open state (PDB code 3J5Q-D). In this proposed model, interaction of the negatively charged phosphate group with the Lys-710 residue (green) in the proximal C terminus of TRPV1 would lead to the open conformational change by promoting movement of the C-helix. The docked structure of LPA 18:1 (cyan) is shown in both the closed and open states.