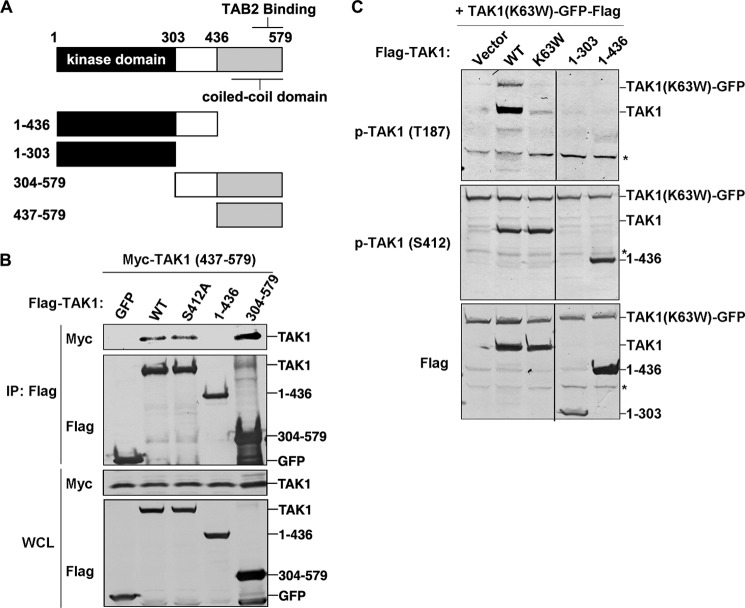
FIGURE 3.
The C-terminal coiled-coil domain of TAK1 is essential for TAK1 intermolecular interaction and autophosphorylation. A, domain organization of mouse TAK1 and the truncation mutants. The kinase and putative coiled-coil domains are indicated. B, 500 ng of FLAG-tagged GFP or TAK1 WT, S412A, or aa 1–436 or 304–579 truncation mutant vector was transfected with 500 ng of Myc-tagged TAK1 aa 437–579 truncation mutant vector into 293T cells for 36 h and then subjected to immunoprecipitation by using M2 FLAG antibody-agarose beads. Immunoprecipitated proteins and whole cell lysates (WCL) were analyzed with anti-FLAG or anti-Myc antibody. C, 500 ng of FLAG-tagged TAK1 WT or its mutants K63W, T187A, S412A, or S412D or the truncation mutant (aa 1–303 or 1–436) vector was co-transfected with 500 ng of TAK1-K63W-GFP-FLAG vector into 293T cells. After 36 h, cell lysates were used for immunoblotting with the indicated antibodies. *, nonspecific bands. IP, immunoprecipitation; p-, phosphorylated.