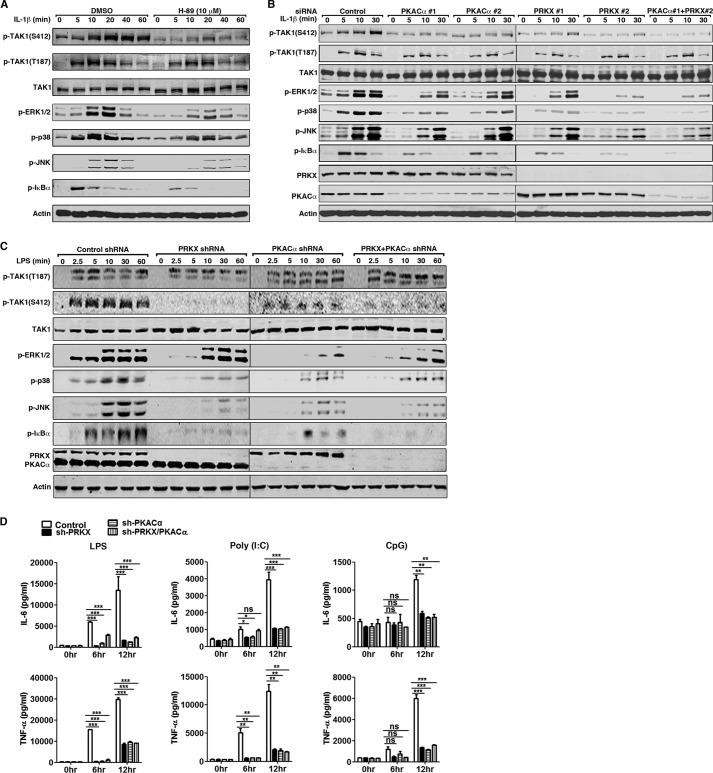
FIGURE 6.
PKACα and PRKX regulate TAK1 kinase activity by phosphorylating TAK1 Ser-412. A, HeLa cells were treated with DMSO or H-89 (10 μm) for 1 h and then stimulated with IL-1β (10 ng/ml) for the indicated time. Cell lysates were subjected to immunoblotting with the indicated antibodies. B, HeLa cells were transfected with scrambled siRNA, two distinct siRNAs against human PRKX or PKACα (40 nm final siRNA concentration) for 48 h, and stimulated with IL-1β (10 ng/ml) for the indicated time. Cell lysates were subjected to immunoblotting with the indicated antibodies. C, stable RAW264.7 cells harboring control shRNA or shRNA against PRKX, PKACα, or both PRKX and PKACα were stimulated with LPS (100 ng/ml) for the indicated time. Cell lysates were collected and used for immunoblotting with the indicated antibodies. D, as in C, RAW264.7 cells were treated with LPS (100 ng/ml), poly(I:C) (20 μg/ml), or CpG ODN (0.3 μm) for 6 or 12 h. Secretion of IL-6 and TNF-α in the supernatant was analyzed by ELISA. Data are plotted as means ± S.E. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus controls. ns, no significance. All experiments were performed at least three times. p-, phosphorylated.