FIGURE 2.
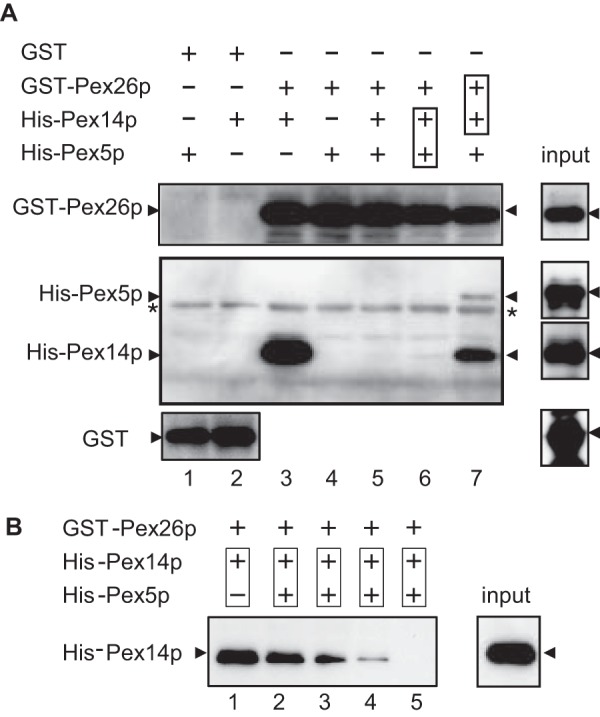
Ternary complex formation of Pex26p, Pex14p, and Pex5p. A, direct binding of Pex26p to Pex14p was assayed with GST (1.0 μg each, lanes 1 and 2), GST-Pex26p (0.5 μg each, lanes 3–7), His6-Pex14p (2.0 μg each, lanes 2, 3, and 5–7), and His6-Pex5pL (3.0 μg each, lanes 1 and 4–7). Purified His6-Pex14p and His6-Pex5p were separately incubated with glutathione-Sepharose beads conjugated to GST (lanes 1 and 2) or GST-Pex26p (lanes 3–7). Boxes in lanes 6 and 7 designate preincubation of the indicated proteins. GST-Pex26p in lane 6 was added to the preincubated mixture of His6-Pex14p and His6-Pex5p. In lane 7, pre-mixed GST-Pex26p and His6-Pex14p were further incubated with His6-Pex5p. Proteins bound to glutathione-Sepharose beads were assessed by immunoblotting with antibodies to Pex26pC (upper panel), Pex5pN plus Pex14pC (middle panel), and GST (lower panel). Input, 10% input. *, a nonspecific band. B, GST-Pex26p (0.5 μg each, lanes 1–5) was incubated with pre-mixed His6-Pex14p (2.0 μg each, lanes 1–5) and His6-Pex5p. Binding of GST-Pex26p to His6-Pex14p and His6-Pex5p was titrated with Pex5p (lane 2, 0.1 μg; lane 3, 0.2 μg; lane 4, 0.4 μg; lane 5, 0.8 μg). Input, 10% input.
