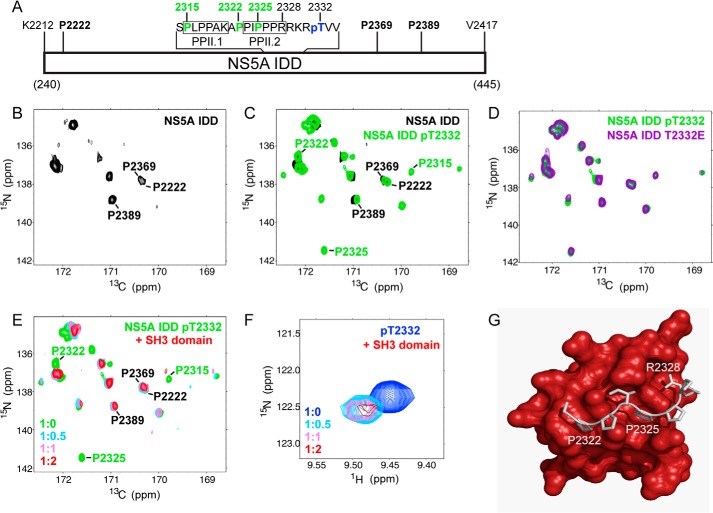
FIGURE 6.
Phosphorylation of Thr-2332 shifts proline residues of the NS5A polyproline motif that contribute to interaction with the SH3 domain of c-Src. A, schematic of NS5A IDD studied by NMR. Proline residues (black, boldface type) were observed in the unphosphorylated IDD. Residues of the two type-II polyproline motifs, PPII.1 and PPII.2, are indicated. Proline residues (green) were observed only in the Thr(P)-2332- and T2332E IDDs. The location of Thr(P)-2332 is indicated. B, prolines in NS5A IDD can be detected by NMR. Shown is the 15N,13C CON spectrum of unphosphorylated NS5A IDD (proline region). Resonances observed represent a very small subset of the proline residues in the IDD. Three were assigned as indicated; none were in PPII. C, phosphorylation of IDD increases the number of proline resonances detected. Shown is the 15N,13C CON spectrum with PKA-phosphorylated IDD (NS5A IDD pT2332; green). The spectrum was overlaid with that of the unphosphorylated IDD (NS5A IDD; black). Many new resonances were observed. Three could be assigned, and these mapped to PPII. D, NS5A IDD T2332E mimics Thr(P)-2332. The spectrum for NS5A IDD T2332E (purple) superimposes with that of NS5A IDD Thr(P)-2332 (green). E, titration of the SH3 domain into NS5A IDD Thr(P)-2332 leads to loss of resonances induced by Thr-2332 phosphorylation. Shown are 15N,13C CON spectra of NS5A IDD Thr(P)-2332 with 0–2 eq of unlabeled c-Src SH3. The absence of red resonances at positions where green resonances are visible indicates a dynamical response to the presence of c-Src. F, chemical shift perturbation of Thr(P)-2332 in response to the presence of c-Src. 1H,15N HSQC spectra are zoomed in on the Thr(P) region for NS5A IDD Thr(P)-2332 with 0–2 eq of unlabeled c-Src SH3. This resonance is also sensitive to the presence of c-Src. G, molecular model of c-Src SH3 domain in complex with NS5A PPII peptide, APPIPPPR. The model was derived from a structure of FYN-SH3 domain in complex with NS5A PPII peptide (43) and structure of c-Src SH3 domain (61). Proline residues whose resonances appear in the presence of phosphorylation and disappear in the presence of c-Src are clearly capable of binding to the protein. The Thr(P)-2332 would be predicted to be close enough to the protein surface for interaction.