FIGURE 4.
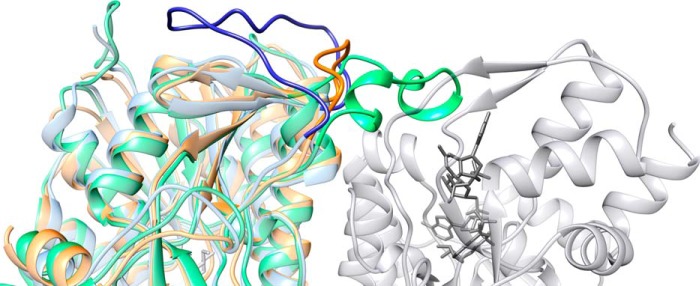
Ribbon representation of the Mg534 structure. Mg534 (orange) superimposed with CapE (green) and FlaA1 (blue) produced using Chimera (43) (University of California, San Francisco). The view is centered on the variable region corresponding to the CapE latch. The molecule in light gray corresponds to the second monomer of the dimer of CapE. The substrate and the coenzyme are colored in dark gray. Whereas the latch of CapE (dark green) associates with the substrate binding domain of the second monomer, the equivalent region of FlaA1 folds back on the first monomer (dark blue). This long loop is absent from the Mg534 structure.
