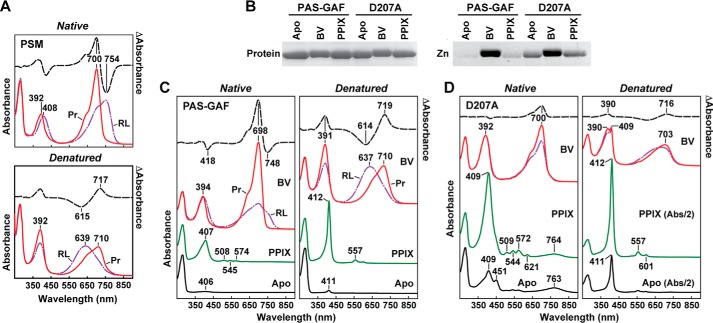
FIGURE 4.
UV-visible absorption and fluorescent properties of the D207A mutation assembled with BV and PPIX. A, absorption spectra before and after acidic denaturation of the PSM of DrBphP assembled with BV. Absorption and difference spectra were recorded for the Pr state or after saturating red-light (RL) irradiation (mostly Pfr) in nondenaturing buffer or after dissolution in 8 m urea (pH 2.0). Absorbance maxima are indicated. Difference spectra (dashed lines) were scaled to 70% of absorption spectra. B, covalent binding of BV and PPIX to the wild-type PAS-GAF fragment and the D207A mutant. Following in vitro assembly, the samples were subjected to SDS-PAGE and either stained for protein with Coomassie Blue (Protein) or assayed for the bound BV/PPIX by zinc-induced fluorescence (Zn). C and D, absorption spectra before and after acidic denaturation of the PAS-GAF fragment from wild-type DrBphP (C) and the D207A mutant (D) assembled with BV or PPIX. Absorption and difference spectra were recorded as in A. Apo, apoprotein before bilin assembly. The scales of the absorption spectra for the denatured PPIX and Apo samples from the D207A mutant were reduced 2-fold for clarity.