FIGURE 6.
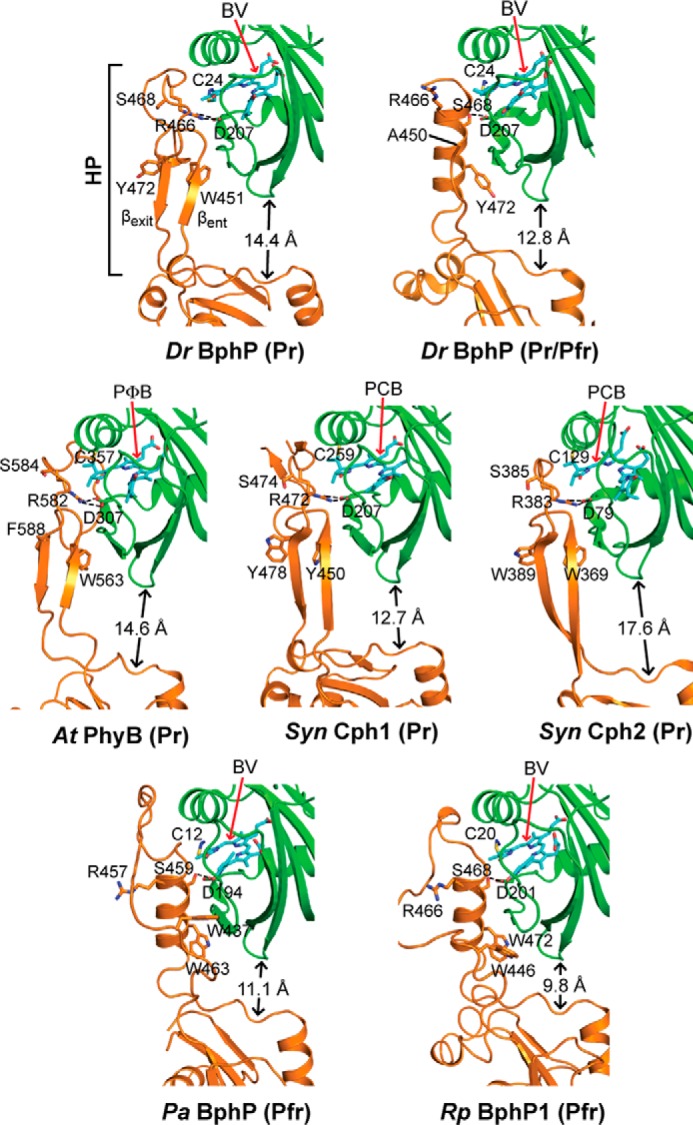
Conformation of the PHY domain hairpin from representative Phys and its interaction with the GAF domain. PSM structures were extracted from D. radiodurans BphP as Pr (PDB code 4Q0J; this report) and a mixed Pr/Pfr state (4O01 (30)), Synechocystis Cph1 as Pr (2VEA (10)), and Cph2 as Pr (4BWI (21)), A. thaliana PhyB as Pr (4OUR (11)), P. aeruginosa BphP as Pfr (3C2W (29)), and R. palustris BphP1 (4GW9 (19)) as Pfr. The GAF and PHY domains are colored in green and orange, respectively, and the bilin is colored in cyan (arrow), the type of which is indicated. PCB, phycocyanobilin. PΦB, phytochromobilin. Side chains are shown for relevant amino acids. Dashed lines indicate hydrogen bond contacts between the DIP (Asp-Ile-Pro) motif aspartate in the GAF domain and either the conserved arginine or serine in the hairpin stem. The distance separating the GAF and PHY domain globular regions is indicated. The distances were measured from the loop separating the β1 and β2 strands of the GAF domain and the α-carbon of a conserved tryptophan (Trp-483 in DrBphP) just proximal to the exiting α-helix of the PHY domain. HP, hairpin. βent and βexit label the entrance (N-terminal) and exit (C-terminal) β-strands in the hairpin.
