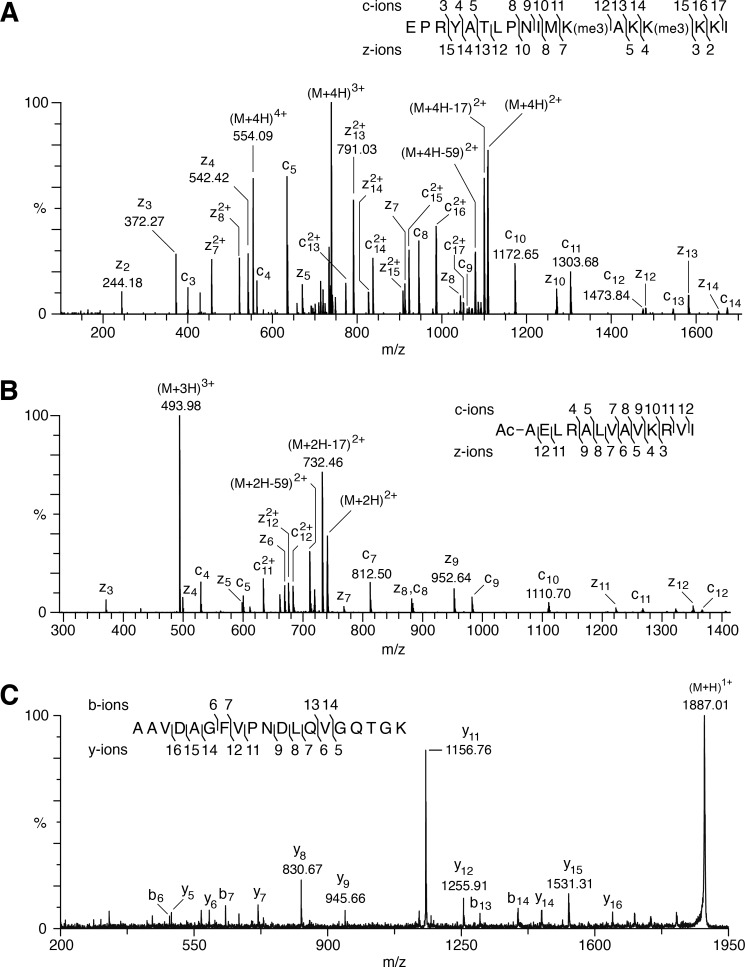
FIGURE 3.
Post-translational modifications of bovine ETFβ and the presence of proline at position 239 of bovine ETFα. A, ETD fragmentation mass spectrum of peptide DN-(188–205) of ETFβ from bovine heart mitochondria, with series of c ions and z ions generated from the [M + 4H]4+ precursor ion, m/z 554.09, mapped onto the sequence of the peptide. B, sequence of the N-terminal peptide of bovine ETFβ. An ETD fragmentation mass spectrum is shown of a triply charged ion, m/z 493.98, generated by cleavage of the protein with AspN. In the inset, the series of c and z fragment ions identified the peptide as the N-terminal fragment, and demonstrated that the α-amino group of Ala-1 is acetylated. C, a MALDI-TOF-TOF fragmentation mass spectrum is shown of a singly charged ion, m/z 1886.96, of a tryptic peptide from the bovine ETF α-subunit. In the inset, the series of b and y fragment ions shows that the peptide corresponds to residues 231–249, and the y9 and y11 ions define the sequence Pro-Asn, and demonstrates the presence of proline and not threonine at position 239.