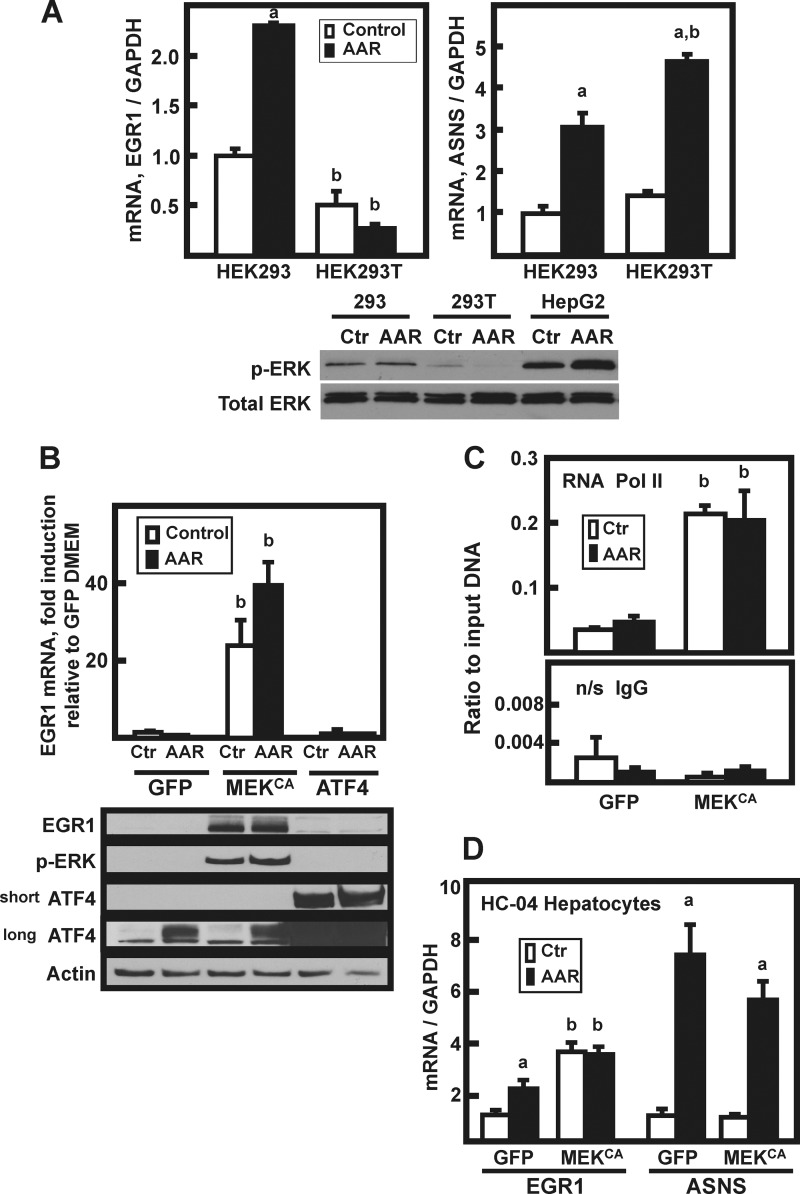
FIGURE 4.
MEK signaling is both necessary and sufficient for induction of EGR1. A, HEK293 or HEK293T cells were incubated in DMEM (Control or Ctr) or DMEM lacking histidine (AAR) for 8 h, and then EGR1, ASNS, and GAPDH mRNA content was analyzed by qPCR. The data are plotted as the averages ± standard deviations of triplicate samples and are representative of multiple independent experiments. An a indicates that the AAR value is different from the DMEM control at p ≤ 0.05, and a b indicates that the HEK293T value is statistically different from the corresponding value obtained with HEK293 cells p ≤ 0.05. Whole cell extracts were also analyzed by immunoblotting for total ERK and p-ERK protein content. B, HepG2 cells were transiently transfected with plasmids expressing GFP, constitutively active MEK (MEKCA), or ATF4, and 36 h later the cells were incubated for 8 h in DMEM (Ctr) or DMEM + HisOH (AAR). RNA and protein extracts were prepared, and EGR1 mRNA content was analyzed by qPCR. The data are plotted as the averages ± standard deviations of triplicate samples and are representative of multiple independent experiments. A b indicates that the value is different from the corresponding GFP-transfected control (GFP) value at p ≤ 0.05. The whole cell extracts were analyzed by immunoblotting for changes in EGR1, p-ERK, ATF4, and actin protein content. Two different exposures (“short” or “long”) of the ATF4 blot are shown to illustrate the relative abundance of endogenous versus overexpressed protein. C, HepG2 cells were transiently transfected with (GFP) or constitutively active MEK (MEKCA), and the AAR was induced as described for B. Following activation of the AAR, the cells were subjected to ChIP analysis for RNA Pol II binding to the EGR1 promoter. The primer sequences used are listed in Table 1. A nonspecific IgG antibody was used as the negative control. The results are given as the averages ± standard deviations for at least three samples, and a b denotes a significant difference of p ≤ 0.05 relative to the corresponding GFP-transfected control cell value. D, nontransformed HC-04 human hepatocytes were transiently transfected with GFP or constitutively active MEK (MEKCA) and treated with HisOH, as described for HepG2 cells in B. The mRNA levels of EGR1, ASNS, and GAPDH were measured by qPCR, and the data were normalized relative to the GFP DMEM value for either EGR1 or ASNS. The results are given as the averages ± standard deviations for at least three samples. An a indicates that the AAR value is different from the DMEM control at p ≤ 0.05, and a b indicates a significant difference of p ≤ 0.05 relative to the corresponding GFP-transfected control cell value.