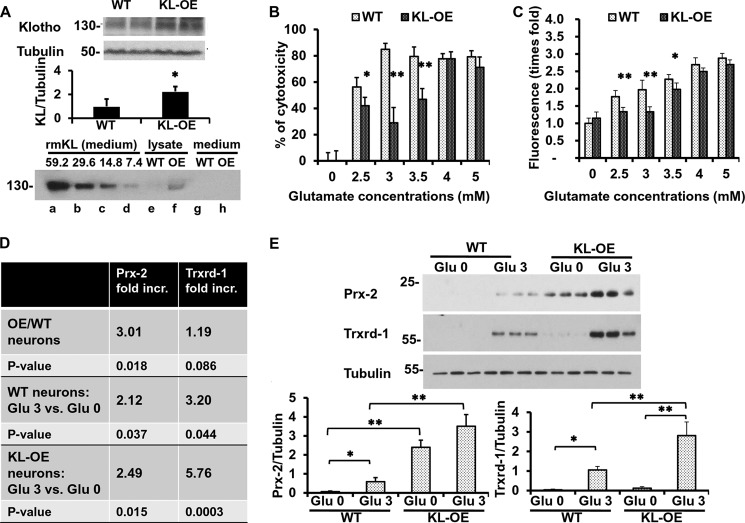
FIGURE 7.
Hippocampal neurons obtained from KL-OE embryos are more resistant to glutamate cytotoxicity than neurons from WT embryos. A, top, WB analysis of Klotho expression in 50 μg of whole brain lysate from WT and KL-OE E18 embryos (n = 4 for each group). Bottom panel, WB analysis of Klotho expression in 20 μl of medium containing serial dilutions of recombinant mouse Klotho (× 10−6 nmol), starting at a concentration of 0.4 μg/ml, which corresponds to 59.2 × 10−6 nmol of Klotho (lanes a–d), 25 μg of cell lysate (lanes e and f), and 20 μl of medium (lanes g and h) collected from hippocampal neurons from WT and KL-OE embryos. B and C, mouse primary hippocampal neurons from WT and KL-OE embryos were treated with glutamate at the indicated concentrations. Twenty-four (B) or eight (C) hours later, the percentage cytotoxicity was assessed using CellTiter Glo, and the accumulation of ROS was measured by 2′,7′-dichlorofluorescein diacetate (B and C, respectively). Error bars, S.D. Each experiment was repeated at least three times, and representative results are shown. D, qRT-PCR analysis of the expression of Prx-2 and Trxrd-1 in the presence or absence of glutamate (3 mm), 24 h after the treatment. The results are presented as -fold increase of groups. First panel, neurons from KL-OE embryos versus neurons from WT embryos in the absence of oxidative conditions; second panel, neurons from WT embryos treated with glutamate (3 mm) versus untreated; third panel, neurons from KL-OE embryos treated with glutamate (3 mm) versus untreated. E, WB analysis of the expression of Prx-2 and Trxrd-1 in the presence or absence of glutamate (3 mm), 24 h after the treatment of hippocampal neurons obtained from WT and KL-OE embryos. Asterisks indicate statistical significance: *, p < 0.05; **, p < 0.01 by t test. Error bars, S.D. Each experiment was repeated three times, and representative results are shown.