Abstract
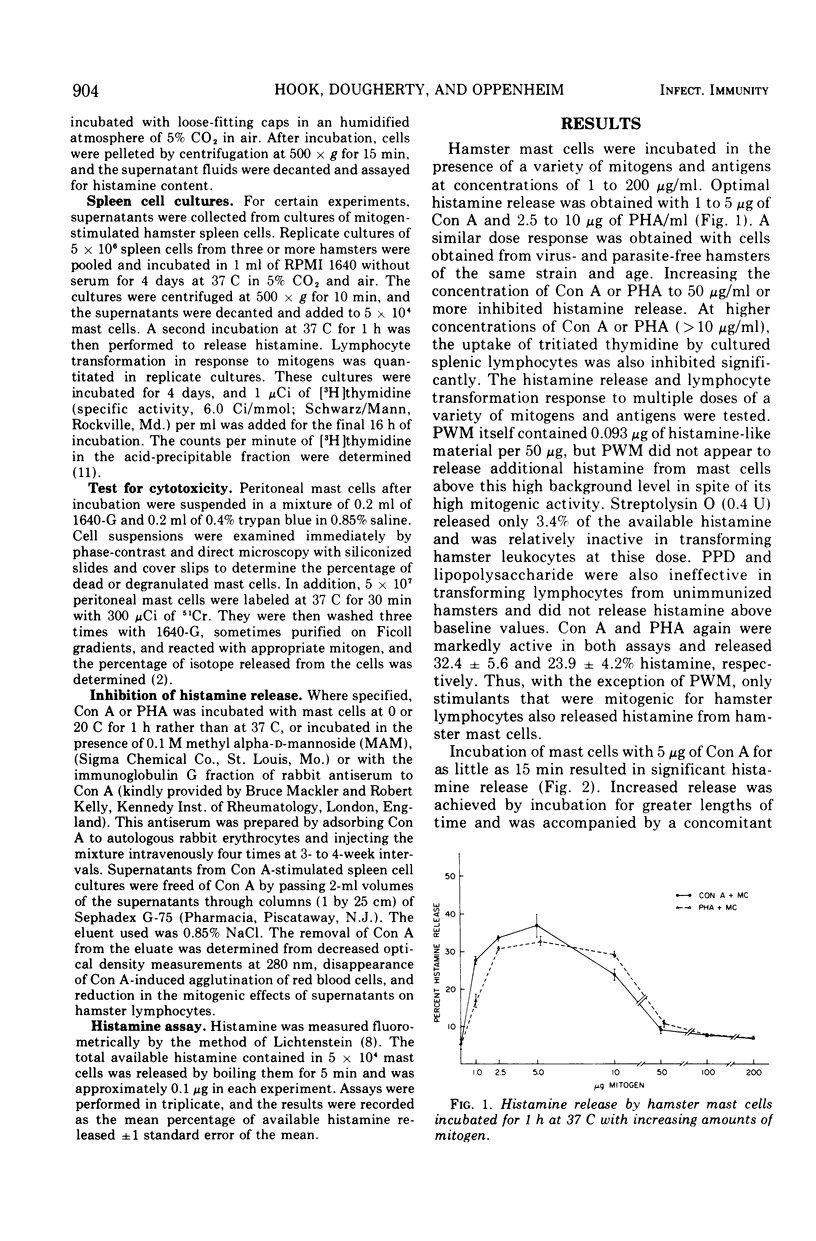
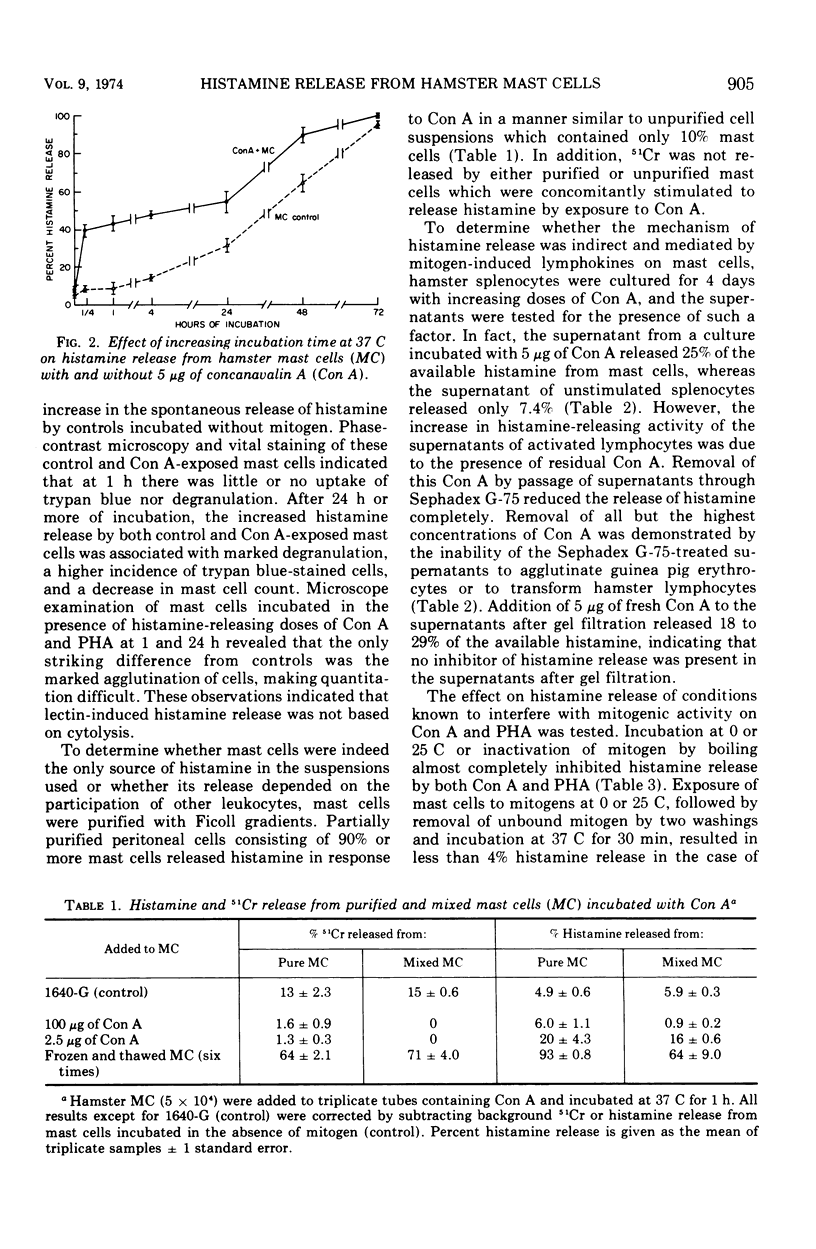
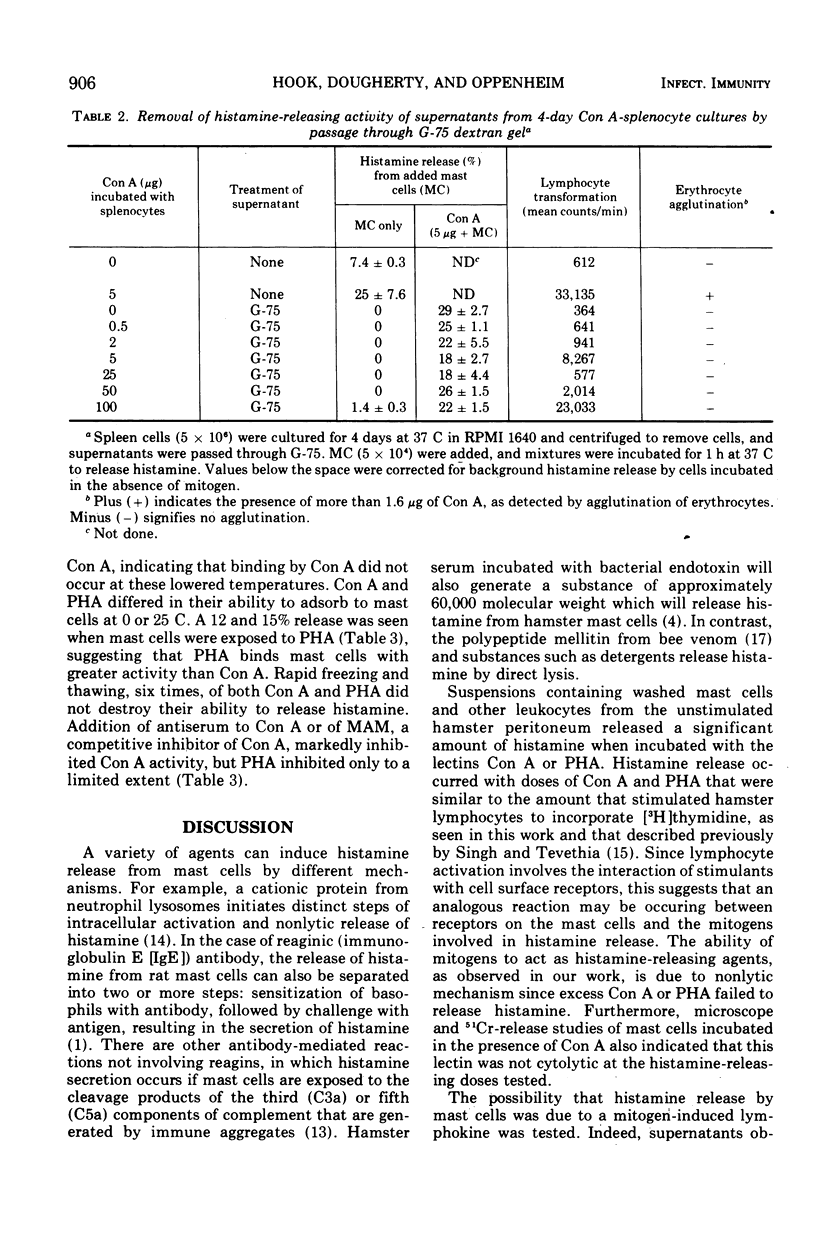
Concanavalin A (Con A) and phytohemagglutinin (PHA) released histamine from hamster mast cells incubated in a serum-free medium. Concentrations of Con A and PHA approximating those optimal for transforming lymphocytes also released maximal amounts of histamine without apparent cytotoxicity. Higher concentrations of mitogen inhibited both lymphocyte transformation and histamine release. Incubation at 37 C for 15 min released histamine, although longer times were more effective. Supernatants from cultured hamster splenocytes stimulated with Con A also released histamine from added mast cells. However, the effect could be inhibited by the addition of 0.1 M methyl alpha-D-mannoside or by passing the spleen cell culture supernatants through Sephadex G-75 to remove Con A. This mitogen-induced release of mast cell histamine is therefore not mediated by a lymphokine but probably results from a direct interaction of mitogens with receptors on mast cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. K., Bloch K. J., Austen K. F. IgE and IgGa antibody-mediated release of histamine from rat peritoneal cells. I. Optimum conditions for in vitro preparation of target cells with antibody and and challenge with antigen. J Exp Med. 1971 Apr 1;133(4):752–771. doi: 10.1084/jem.133.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty T. G., Wunderlich J. R. Quantitative in vitro assay of cytotoxic cellular immunity. J Natl Cancer Inst. 1970 Oct;45(4):761–772. [PubMed] [Google Scholar]
- Hook W. A., Snyderman R., Mergenhagen S. E. Further characterization of a factor from endotoxin-treated serum which releases histamine and heparin from mast cells. Infect Immun. 1972 Jun;5(6):909–914. doi: 10.1128/iai.5.6.909-914.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook W. A., Snyderman R., Mergenhagen S. E. Histamine-releasing factor generated by the interaction of endotoxin with hamster serum. Infect Immun. 1970 Oct;2(4):462–467. doi: 10.1128/iai.2.4.462-467.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Johansson S. G., Bennich H. Histamine release from human leukocytes by anti-gamma E antibodies. J Immunol. 1969 Apr;102(4):884–892. [PubMed] [Google Scholar]
- Keller R. Concanavalin A, a model "antigen" for the in vitro detection of cell-bound reaginic antibody in the rat. Clin Exp Immunol. 1973 Jan;13(1):139–147. [PMC free article] [PubMed] [Google Scholar]
- Keller R. Interrelations between different types of cells. II. Histamine-release from the mast cells of various species by cationic polypeptides of polymorphonuclear leukocyte lysosomes and other cationic compounds. Int Arch Allergy Appl Immunol. 1968;34(2):139–144. [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M. The immediate allergic response: in vitro separation of antigen activation, decay and histamine release. J Immunol. 1971 Oct;107(4):1122–1130. [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- OSLER A. G., RANDALL H. G., HILL B. M., OVARY Z. Studies on the mechanism of hypersensitivity phenomena. III. The participation of complement in the formation of anaphylatoxin. J Exp Med. 1959 Aug 1;110(2):311–339. doi: 10.1084/jem.110.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Wolstencroft R. A., Gell P. G. Delayed hypersensitivity in the guinea-pig to a protein-hapten conjugate and its relationship to in vitro transformation of lymph node, spleen, thymus and peripheral blood lymphocytes. Immunology. 1967 Jan;12(1):89–102. [PMC free article] [PubMed] [Google Scholar]
- Powell A. E., Leon M. A. Reversible interaction of human lymphocytes with the mitogen concanavalin A. Exp Cell Res. 1970 Oct;62(2):315–325. doi: 10.1016/0014-4827(70)90560-4. [DOI] [PubMed] [Google Scholar]
- Ranadive N. S., Cochrane C. G. Mechanism of histamine release from mast cells by cationic protein (band 2) from neutrophil lysosomes. J Immunol. 1971 Feb;106(2):506–516. [PubMed] [Google Scholar]
- Singh S. B., Tevethia S. S. In vitro stimulation of hamster lymphocytes with concanavalin A. Infect Immun. 1972 Mar;5(3):339–345. doi: 10.1128/iai.5.3.339-345.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UVNAS B., THON I. L. Isolation of "biologically intact" mast cells. Exp Cell Res. 1959 Nov;18:512–520. doi: 10.1016/0014-4827(59)90316-7. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Bell R. M. Membrane matrix disruption by melittin. Biochim Biophys Acta. 1972 Nov 2;288(2):255–262. doi: 10.1016/0005-2736(72)90246-5. [DOI] [PubMed] [Google Scholar]


