Abstract
Through free radical-mediated peroxidation, cyclooxygenase (COX) can metabolize dihomo-γ-linolenic acid (DGLA) and arachidonic acid(AA) to form well-known bioactive metabolites, namely, the 1-series of prostaglandins (PGs1) and 2-series of prostaglandins(PGs2), respectively. Unlike PGs2, which are generally viewed as pro-inflammatory and pro-carcinogenic PGs, PGs1 may possess anti-inflammatory and anti-cancer activity. Previous studies using ovine COX along with spin trapping and the LC/ESR/MS technique have shown that certain exclusive free radicals are generated from different free radical reactions in DGLA and AA peroxidation. However, it has been unclear whether the differences were associated with the contrasting bioactivity of DGLA vs. AA. The aim of this study was to refine the LC/MS and spin-trapping technique to make it possible for the association between free radicals and cancer cell growth to be directly tested. Using a colon cancer cell line, HCA-7 colony 29, and LC/MS along with a solid phase extraction, we were able to characterize the reduced forms of radical adducts (hydroxylamines) as the free radicals generated from cellular COX-catalyzed peroxidation. For the first time, free radicals formed in the COX-catalyzed peroxidation of AA vs. DGLA and their association with cancer cell growth was assessed (cell proliferation via MTS and cell cycle distribution via PI staining) in the same experimental setting. The exclusive free radicals formed from the COX-catalyzed peroxidation of AA and DGLA were shown to be correlated with the cell growth response. Our results indicate that free radicals generated from the distinct radical reactions in COX-catalyzed peroxidation may represent the novel metabolites of AA and DGLA that correspond to their contrasting bioactivity.
Keywords: Cell growth response, COX catalyzed peroxidation, ESR spin-trapping, free radicals, HCA-7 colony 29 cells, LC/MS and LC/MS2
INTRODUCTION
Cyclooxygenase (COX) is an enzyme that catalyzes peroxidation of dietary and membrane polyunsaturated fatty acids (PUFAs). COX exists as two isoforms, the constitutive isoform COX-1 and the inducible isoform COX-2 [1–2]. Unlike COX-1, which is expressed in nearly all mammalian tissues, COX-2 is often up-regulated at inflammatory sites and in various tumor tissues, and thus has received much research attention for its role in inflammation-derived diseases and various cancers [3–8]. It was reported that COX-2 is over expressed ~40% in human adenomas and ~80% in adenocarcinomas relative to normal mucosa in colon tissues [9–10].
Arachidonic acid (AA, 20:4), a major PUFA in mammalian cells, and its upstream PUFA, dihomo-γ-linolenic acid (DGLA, 20:3), are both long-chain ω-6 PUFAs and the substrates of COX. A high intake of ω-6 PUFAs was found to enhance tumorigenesis and metastasis in the development of colon, breast, and prostate cancers [11–16] presumably via COX-catalyzed AA peroxidation to generate pro-inflammatory and pro-carcinogenic 2-series prostaglandins (PGs2). Prostaglandin E2 (PGE2), a member of PGs2, is the major AA-derived PG from COX. PGE2could stimulate colorectal carcinogenesis by activating the pro-survival signaling pathways, including extracellular-signal-regulated kinases, cyclic adenosine monophosphate/protein kinase A, and epidermal growth factor receptor [17–19].
Unlike its downstream PUFAs (e.g. AA), however, DGLA may possess anti-inflammatory and anti-cancer activities by virtue of increasing the production of the 1-series of prostaglandins (PGs1) through its COX-catalyzed peroxidation. For example, prostaglandin E1 (PGE1)was reported to inhibit vascular smooth muscle cell proliferation, reduce vascular cell adhesion, and attenuate the development of atherosclerosis [20–23]. PGE1 also exerted an inhibitory effect on the growth of melanoma cells and HeLa cells [24–25].
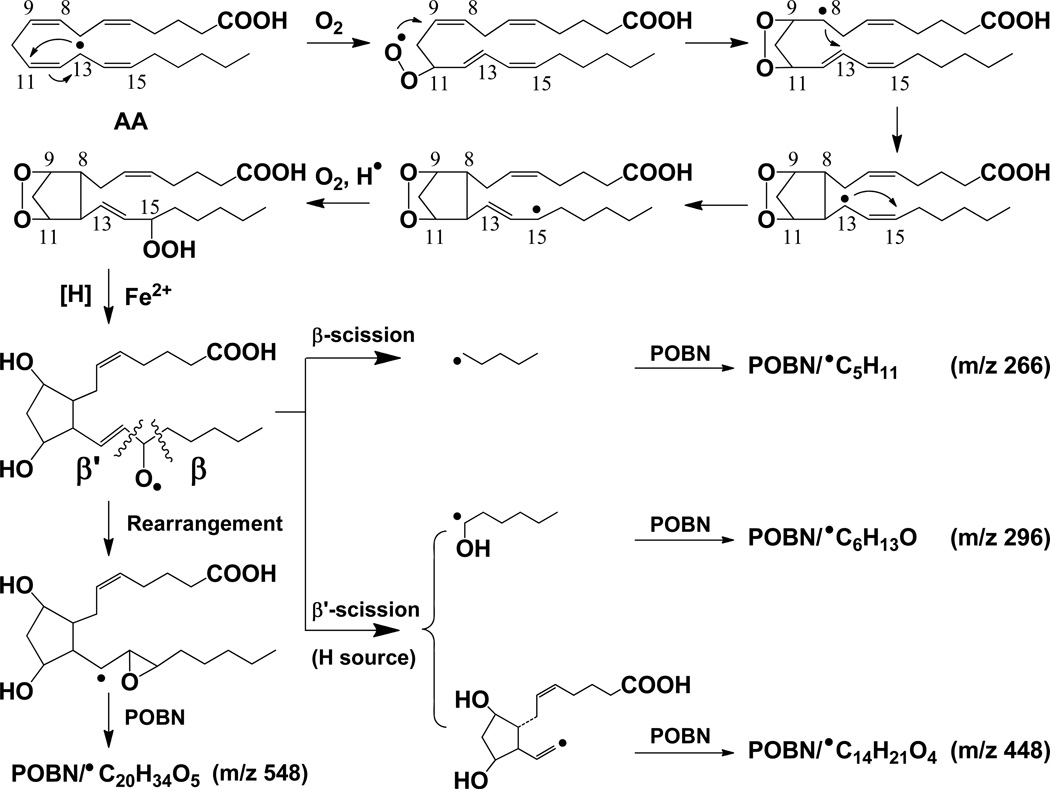
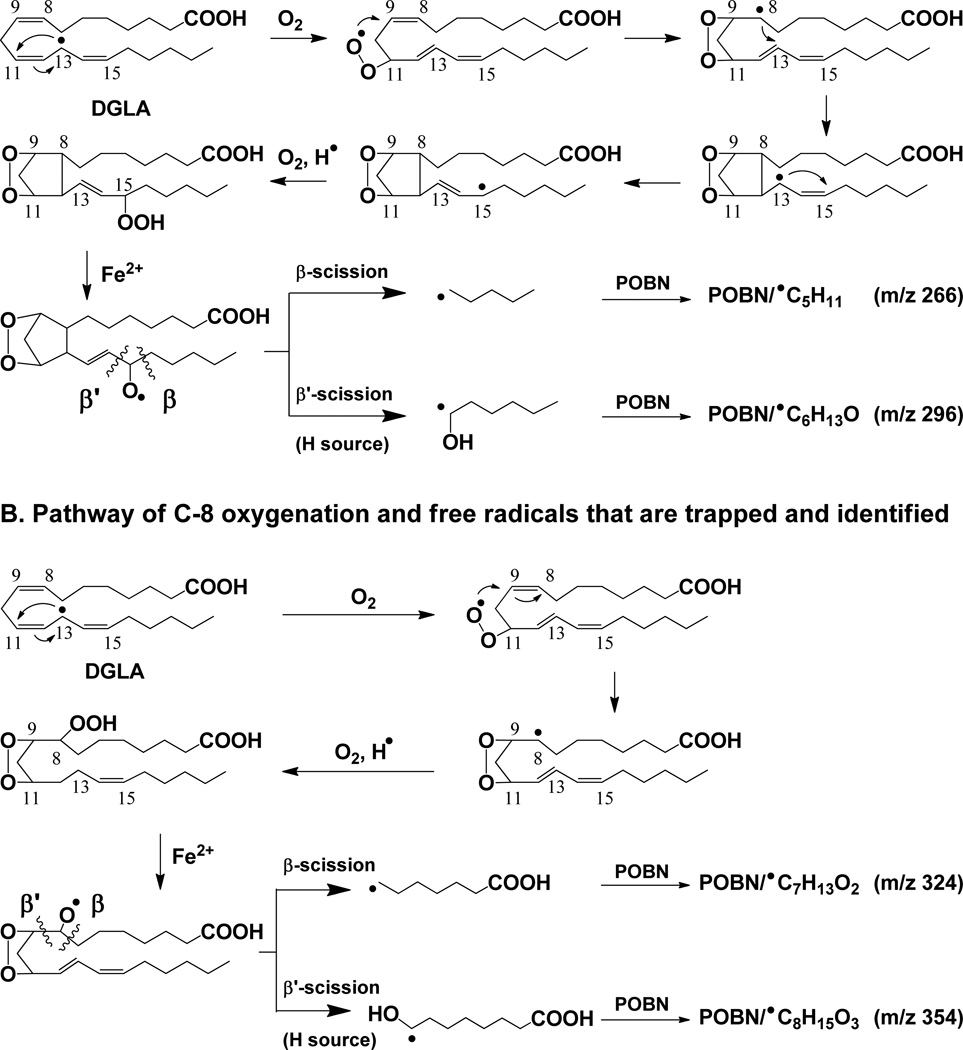
It is well-known that COX metabolizes AA and DGLA through a series of free radical reactions [26]. As the most reactive intermediates, however, the AA- and DGLA-derived free radicals formed from COX-catalyzed peroxidation have never been identified and characterized until a novel technique, the combined technique of spin-trapping and LC/ESR/MS, was recently developed [27–32]. According to the free radicals that have been characterized in COX-catalyzed AA and DGLA peroxidation using an ovine COX-2 enzyme (cell-free) in vitro system [29, 32], some pathways of AA and DGLA oxidation could be similar to each other while others occur for only one of these substrates. In the free radical reactions that are due to the shared structural moiety (C-8 to C-20) of AA and DGLA, some identical or analogous free radicals are formed from C-15 oxygenation (Schemes 1–2). However, unique carbon-carbon double bond (●C=C) free radicals were formed only in COX-catalyzed AA peroxidation; no similar free radical could be detected in C-15 oxygenation of DGLA [29, 32]. In addition, DGLA can also be oxidized by C-8 oxygenation due to its C-1 to C-7 structural moiety, which is different from the corresponding section of AA. The C-8 oxygenation thus leads to the formation of two exclusive DGLA-derived free radicals from its COX-catalyzed peroxidation(Scheme 2B) [32].
Scheme 1.
Proposed mechanism of COX-catalyzed AA peroxidation formation of free radicals via C-15 oxygenation [29]. Free radical reactions include the formation of C-13 radicals, C-9/C-11 endoperoxide bridge (adding the first O2 via the cyclooxygenase activity of COX), C-8 and C-12 cyclization, and C-15 oxygenation (adding the second O2 via the peroxidase activity of COX). Four types of free radicals, ●C5H11, ●C6H13O, ●C14H21O4 and ●C20H34O5, are listed as formed and trapped by POBN as m/z 266, m/z 296, m/z 448 and m/z 548 ions, respectively, in LC/MS. Note when β’-scission takes place at the PGF2 stage,1, 5 intra-molecular H abstraction results in formation of both ●C14H21O4 and ●C6H13O [29]. However, when β’-scission takes place at the PGH2 stage, ●C6H13O would be the only radical product as alternative cleavage pathway. Hexanol (a derivative of ●C6H13O) was detected in COX/AA systems by GC/MS (data not shown) from cellular experiments in which POBN was absent.
Scheme 2.
Proposed mechanism of COX-catalyzed DGLA peroxidation. (A) Formation of free radicals via C-15 oxygenation (same as described in Scheme 1, COX-catalyzed AA peroxidation). Thus, two radicals common to DGLA and AA peroxidation, ●C5H11 and ●C6H13O, were formed and trapped by POBN as m/z 266 and 296 ions, respectively, in LC/MS; and (B) Formation of free radicals via C-8 oxygenation, another way to add a second O2 onto DGLA followed by formation of the C-13 radical and C-9/C-11 endoperoxide bridge. Thus, two exclusive free radicals, ●C7H13O2 and ●C8H15O3, were formed and trapped by POBN as m/z 325 and m/z 355 ions, respectively, in LC/MS. Note that the β’-scission of a PGH1-type alkoxyl radical only forms ●C6H13O; no ●C=C radical forms from DGLA [32] as found in AA peroxidation. Hexanol (a derivative of ●C6H13O) was also detected in COX/DGLA systems by GC/MS (data not shown) from cellular experiments in which POBN was absent.
In order to investigate the possible association between cancer cell growth and exclusive free radicals generated from COX-catalyzed AA vs. DGLA peroxidation, we made the first effort in this study to characterize the free radicals formed from a human colon cancer cell line (i.e., HCA-7 colony 29, the cells that express high COX-2). Our refined LC/MS technique along with solid phase extraction (SPE) allowed us for the first time to profile free radicals under normal cell growth conditions in which the cell’s growth responses could also be assessed in the same experimental setting and at the same time points. We accomplished this profiling by monitoring reduced POBN radical adducts (e.g.. hydroxylamines, Scheme 3), with their structures confirmed by both dual spin-trapping and LC/MS2 analysis. This study thus provides a new way to screen free radicals generated in cells, particularly under normal cell culture conditions. Our new protocol allowed us to directly study the free radical-associated PUFAs’ bioactivity, thus improving our understanding of COX-catalyzed lipid peroxidation in cancer biology.
Scheme 3.
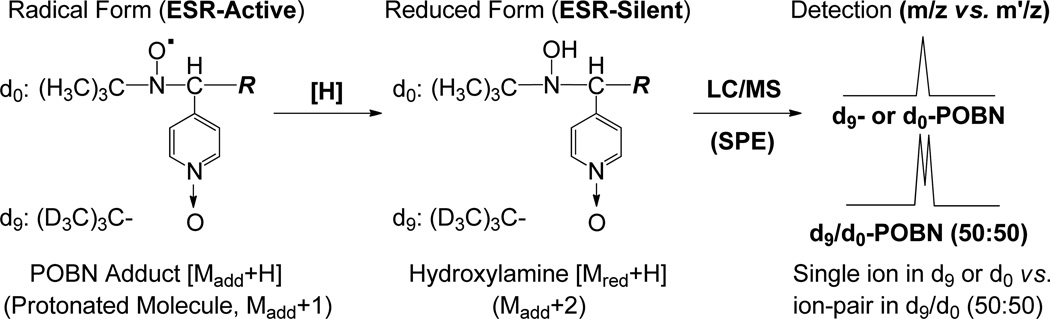
Reduction of POBN-radical adducts (ESR-active) to form the corresponding hydroxylamines (ESR-silent, but MS-active). Hydroxylamines are a much more stable redox form of radical adducts compared to POBN adducts in radical form (ESR-active). The dual spin-trapping technique allowed us to readily single out hydroxylamines as ion pairs, each with a 9-Da difference in LC/MS [32].
MATERIALS AND METHODS
Reagents and Cell Line
Ammonia hydroxide (NH4OH, 5N), ethyl acetate, glacial acetic acid (HOAc), heptanoic acid, 1-hexanol, formic acid, 8-hydroxyoctanoic acid, and ribonuclease A (from bovine pancreas)were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Acetonitrile (ACN, HPLC grade), water (H2O, HPLC grade) and ethyl alcohol (200 proof) were purchased from EMD Chemicals (Gibbstown, NJ, USA). Propidium iodide was purchased from Life Technologies (Grand Island, NY, USA). AA and DGLA were purchased from Nu-Chek-Prep (Elysian, MN, USA). Deuterated AA and DGLA (e.g. AA-d8and DGLA-d6), deuterated PGE standard (e.g. PGE1-d4 and PGE2-d9), and CP-24879 (Δ-5 desaturase inhibitor) were obtained from Cayman Chemicals (Ann Arbor, MI, USA). The high-purity spin trap agent α-[4-pyridyl-1-oxide]-N-tert-butyl nitrone (POBN) was purchased from Alexis Biochemicals (San Diego, CA, USA). Deuterated POBN (d9-POBN) was synthesized in our laboratory with help from the Core Synthesis Facility, Center for Protease Research, North Dakota State University.
The human colon cancer cell line, HCA-7 colony 29, was purchased from the European Collection of Cell Cultures (Porton Down, Salisbury, UK). Fetal bovine serum (FBS) and trypsin-EDTA were obtained from Thermo Fisher Scientific (Logan, UT, USA). DMEM high glucose medium (phenol-red free) was obtained from Scien Cell Research Laboratories (Carlsbad, CA, USA). The Cell Titer 96® AQueous One Solution Cell Proliferation Assay kit was purchased from Promega (Madison, WI, USA).
Cellular Spin-Trapping Experiment
Spin-trapping experiment with cell suspension in phosphate-buffered saline (PBS)
HCA-7 colony 29 cells were grown in DMEM high glucose medium (phenol red-free) supplemented with 10% FBS in an incubator containing a 95% humidified atmosphere of 5% CO2 at 37°C. To measure the ESR-active free radical spin adducts, HCA-7 colony 29 cells at ~90% confluence were trypsinized, harvested, and suspended in 200 µL PBS(~108 cells/mL). POBN and PUFAs at final concentrations of 50 mM and 1.0mM, respectively, were then added to the cell-PBS suspension to start the COX-catalyzed PUFA peroxidation and POBN spin-trapping reaction. This complete reaction mixture was incubated at 37°C in the absence of light, and mixed with ACN (1:1, v/v) at 30 min to stop the reaction. After centrifuging 15 min at 14,000 rpm with an Eppendorf centrifuge (Microfuge 22R), supernatant was collected and condensed to an ACN-free solution for LC/ESR and LC/MS analysis.
Spin-trapping experiment with cells grown under normal culture conditions
HCA-7 colony 29 cells were cultured in DMEM high glucose medium (phenol red-free) supplemented with 10% (v/v) FBS in an incubator containing a 95% humidified atmosphere of 5% CO2 at 37°C. Cells at~30% confluence were seeded at a density of 4×106 cells per 100 mm petri dish. With a one-night incubation to allow the cells to attach well to the dish wall, POBN and PUFAs at final concentrations of 20 mM and 100µM, respectively, were added to start the peroxidation and spin-trapping reaction. Instead of measuring ESR-active radical adducts, we actually measured the reduced form (hydroxylamines, Scheme 3) of POBN adducts by LC/MS and LC/MS2. At time points of 0.5, 4, 8, 12, 24, and 48 h, the cell culture medium together with cells that were scrubbed and homogenized with a Sonifier (150 Branson Ultrasonics) were mixed with ACN (1:1, v/v) to stop the reaction. The mixture solution was then vortexed and centrifuged for 15 min at 3,000 rpm, and the supernatant (~4.0 mL) was subjected to solid phase extraction (SPE) followed by LC/MS and LC/MS2analysis. Dual spin-trapping experiments and measurement of hydroxylamines from both d0/d9POBN adducts in cells were conducted the same way as the POBN spin-trapping experiments described above except that (50:50) d0/d9 POBN (20 mM) was added to the cell culture media.
Solid Phase Extraction, LC/MS and LC/MS2 Analysis of Hydroxylamines
A mixed-mode anion exchange SPE cartridge (Oasis MAX, Waters, MA) was employed to extract and condense the hydroxylamines due to the charge of the pyridyl-oxide group of the reduced POBN adduct. A MAX SPE cartridge was preconditioned with 2.0 mL methanol and 2.0mL water; then 4.0 mL of the mentioned supernatant was loaded and washed by 1.0 mL 5% NH4OH and 1.0 mL methanol. Hydroxylamines in the supernatant were then eluted with 2.0mL ACN:MeOH (60:40, v/v; 2% formic acid). The elution was condensed completely with an Eppendorf concentrator (Vacufuge™) and then reconstituted in 100 µL MeOH:H2O (50:50, v/v) for LC/MS analysis.
The LC/MS system consisted of an Agilent 1200 series HPLC system and an Agilent 6300 LC/MSD SL ion trap mass system. HPLC separations were performed on a C18 column (Zorbax Eclipse-XDB, 4.6×75 mm, 3.5 µm) equilibrated with 90% A (H2O-0.1% HOAc) and 10% B (ACN-0.1% HOAc). Forty microliters of the condensed and reconstituted sample was injected into the LC/MS system by auto sampler and eluted at a 0.8 mL/min flow rate with a combination of gradient and isocratic elution: (i) 0 – 5 min, 90 to 73% A and 10 to 27% B; (ii) 5–25 min (isocratic), 73% A and 27% B; (iii) 25- 40 min, 73% to 30% A and 27% to 70% B; (iv) 40–43 min, 30% to 5% A and 70% to 95% B; and (v) 43–50 min (isocratic), 5% A and 95% B. The outlet of the UV detector in LC was connected to the MS system with red PEEK tubing (0.005 i.d.) along with a splitter by which the flow rate to MS was adjusted to 0.1 mL/min.
Electro spray ionization in positive mode was used for all measurements of POBN adducts and hydroxylamines. Total ion current (TIC) chromatograms in full mass scan mode (m/z 50 to m/z 600) were performed to profile all products formed in the reaction of COX-catalyzed AA and DGLA in the presence of POBN. Other MS settings were capillary voltage, −4500 V; nebulizer press, 20 psi; dry gas flow rate, 8.0 L/min; dry temperature, 100°C; compound stability, 20%; and number of scans, 50. An extracted ion current (EIC) chromatogram of ions of interest was projected from the total ion current (TIC) chromatogram to determine the number of isomers of given ions. Normally an isolation width of ±0.5 Da was selected for EIC. The multiple reaction monitoring (MRM) mode of LC/MS2 was used to confirm structural assignments of hydroxylamines. A width of ±2.0 Da was typically selected to isolate parent ions of interest. Other MS settings were the same as the scan mode listed above except the number of scans, 5. For quantification of hydroxylamines, a small amount D9-POBN (internal standard as described elsewhere [30–31]) was added to cells and culture media with a stop solution (ACN), and SPE extraction as well as LC/MS analysis was then conducted. The amount of hydroxylamine generated in cellular peroxidation and the spin trapping reaction was normalized to the numbers of cells at different incubation time points, in which total live cells could be significantly different.
LC/MS Analysis of AA, DGLA, and PGEs
Under the same culture conditions and time points as conducted in the cellular spin-trapping experiments, AA, DGLA, PGE1 and PGE2 in the reaction mixture were quantified by LC/MS using their respective internal standards by same preparation procedures:(1) a two milliliter sample of cell culture medium was collected and internal standards (AA-d8, DGLA-d6, PGE1-d4, PGE2-d9)were added to make a total of 3.0 mL of 15% methanol solution; (2) the reaction mixture was vortexed for 1 min, seton ice for 30 min, and centrifuged for 15 min at 3,000 rpm. The supernatant was then collected and the pH was adjusted to 3.0 with 0.2N HCl; (3) the acidified supernatant was loaded on a reverse phase SPE cartridge (SampliQ Silica C18 ODS, Agilent, CA) which was preconditioned by 2.0mL methanol and 2.0 mL water. AA, DGLA and PGEs were then washed with 1.0 mL of water and eluted out with 2.0 mL ethyl acetate; and (4) the elution was completely condensed with an Eppendorf concentrator and reconstituted with 100 µL ethanol for LC/MS analysis.
A five-micro liter sample of such a reconstituted sample was injected into the same LC/MS system as described above. The analyte was eluted with a combination of gradient and isocratic elution: (i) 0–12 min (isocratic), 68% A and 32% B; (ii) 12–14 min, 68% to 44% A and 32% to 56% B; (iii) 14–28 min (isocratic), 44% A and 56% B; (iv) 28 – 30 min, 44% to 14% A and 56% to 86% B; (v) 30 – 38 min, 14% to 5% A and 86% to 95% B; and (vi) 38 – 44 min (isocratic), 5% A and 95% B. Electro spray ionization in negative mode was used for all LC/MS measurements of PUFAs and PGEs. Other MS settings were nebulizer press, 15 psi; dry gas flow rate, 5.0 L/min; dry temperature, 325°C. The concentrations of DGLA, AA and PGE1/PGE2in the samples were quantified as m/z 305, m/z 303, and m/z 353/351 ions with their individual internal standards, e.g. m/z 311 ions as DGLA-d6 and AA-d8and m/z 357vs. m/z 360 as PGE1-d4 vs. PGE2-d9respectively. The amounts of PUFAs and PGEs formed in the cellular peroxidation reaction were also normalized to the numbers of live cells at different incubation times.
Cell Proliferation and Cell Cycle Distribution Analysis
The cell proliferation assay (MST) was performed with a Cell Titer 96® Aqueous One Solution Cell Proliferation Assay kit according to the manufacturer's instructions. Briefly, cells were seeded at 5,000 cells/well in 96-well plates and treated with 100 µM AA, 100 µM DGLA (with/without 5.0µM Δ-5 desaturase inhibitorCP-24879), 0.01 to 1.0 µM PGEs, and their free radical derivatives (i.e., 1-hexanol, heptanoic acid, or 8-hydroxyoctanoic acid, respectively). After a two-day incubation,20 µL Cell Titer 96 Aqueous One Solution Reagent was added to each well. Following additional 4h incubation with the reagent, the absorbance at 490 nm, representing the quantity of formazan product, which is directly proportional to the number of living cells, was recorded by a 96-well plate reader (SpectraMax M5, Molecular Devices). Cell viability was expressed as a percentage of the control group.
The effect of AA, DGLA (with/without CP-24879), PGEs, and free radical derivatives on cell cycle distribution was examined by flow cytometry after staining the cells with propidium iodide (PI-staining). Briefly, 4 × 106 cells were seeded, incubated overnight, and exposed to AA, DGLA (with/without CP-24879), and PGEs as well as the radical derivatives. At time points of 8, 12, 24 h, cells were trypsinized, washed with PBS, and then fixed in refrigerated 70% ethanol at 4°C for about 30 min, with a final concentration of 1×106 cells/mL. The cells were then treated with 10 µL ribonuclease A (10 mg/mL) and 400 µL propidium iodide (50 µg/mL). After 30 min, the cell cycle distribution was measured by an Accuri C6 Flow Cytometer (BD, NJ). The percentage of cells (at least 10,000 cells were counted)in different phases of cell cycle was analyzed by Flow Jo software (Tree Star, OR).
Radical Trapping Ability of POBN in Cell Culture Media
The time course (up to 48 h) of the reaction of 20 mM POBN with free radicals, i.e., POBN’s radical trapping ability, was examined with a spin trapping experiment of POBN reacting with hydroxyethyl (●C2H5O). At each experimental time point, POBN-containing culture medium (cell free) was mixed with ethanol (0.2 mM) and Fe2+ (1.0 mM, prepared from ferrous ammonium sulfate in redistilled water with the pH adjusted to ~ 2.5[34]), and directly introduced to an ESR flat cell for ESR analysis. ESR spectra were recorded in magnetic field scans using a Bruker EMX spectrometer equipped with a super high Q cavity operating at 9.78 GHz at room temperature. Other ESR spectrometer settings were magnetic field center, 3497.4 G; magnetic field scan, 70 G; modulation frequency, 100 kHz; microwave power, 20 mW; modulation amplitude, 1.0 G; receiver gain, 5.0×104; time constant, 0.655 s; and conversion time, 0.164 s.
RESULTS
Measurement of ESR-Active Free Radical Adducts
When offline ESR (magnetic field scan) was used to measure free radical adducts from cells (HCA-7 colony 29) incubated in PBS and in culture media in the presence of POBN and AA/DGLA, a six-line ESR signal of POBN radical adducts (aN≈ 15.4 G and aH≈ 2.5 G) could be observed in the cell-PBS system, but no ESR signal was observed from cells grown in culture media (data not shown). When the combined technique of LC/ESR/MS was used to further analyze more specific free radical information from the offline ESR signal, many free radical spin adducts, including isomers, were recognized as those having previously been characterized via LC/MS in a (cell-free)COX system [29, 32].
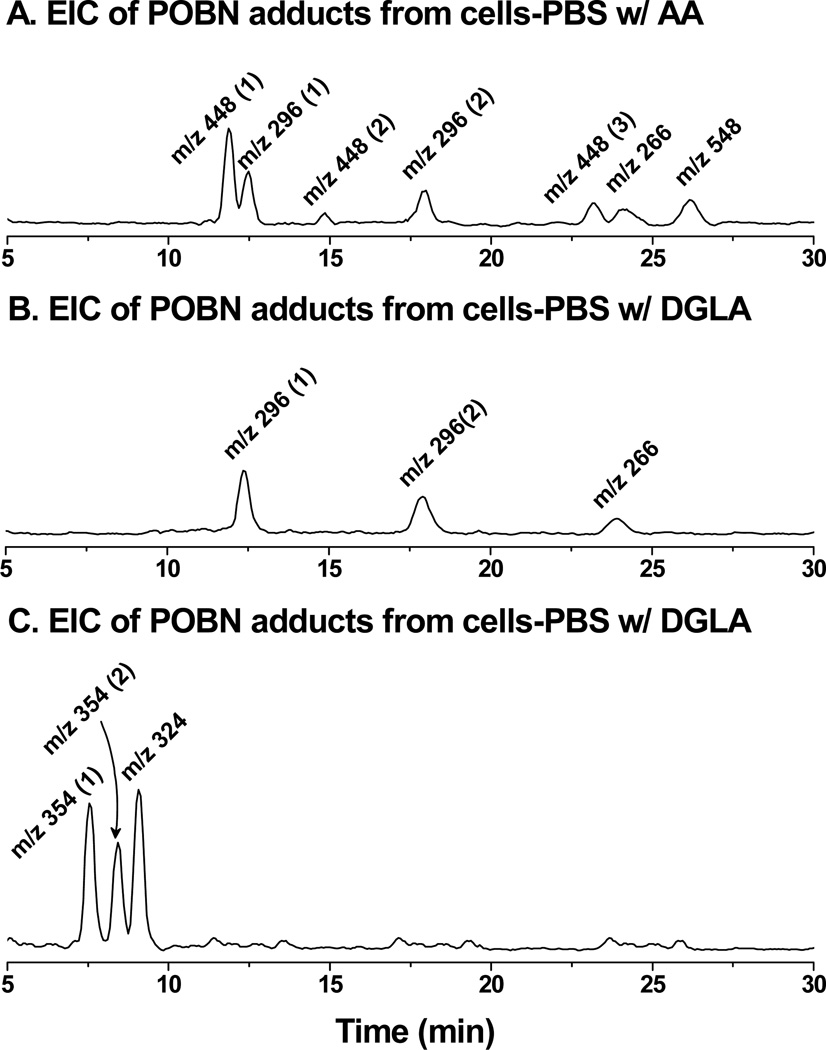
To complete a cellular COX-catalyzed peroxidation and spin-trapping experiment in PBS in 30 min, the length of time cells could survive the conditions, high doses of AA/DGLA (≥ 500 µM) and POBN (≥ 50 mM) were needed. Although no good quality LC/ESR chromatogram was obtained (data not shown), an EIC chromatogram projected from the full LC/MS scan (m/z 50 to m/z 600) clearly demonstrated that POBN adduct molecules of m/z 266, m/z 296, m/z 448, and m/z 548 were formed in COX-catalyzed AA peroxidation (Figure 1A). The EIC peak of the m/z 266 ion corresponded to the POBN spin adduct of pentyl radicals(POBN/●C5H11tR≈ 24.0 min) that could form from β-scission of the PGF2-type alkoxyl radical (Scheme 1). The two EIC peaks of m/z 296appeared to correspond to the two isomers of the POBN spin adduct of hexanol radicals (POBN/●C6H13O, tR≈ 12.4 and 17.8 min). Hexanol radical was believed to be generated in a special β-scission (e.g. β’-scission of PGF2-type alkoxyl radical, Scheme 1) from which three isomers of unique ●C=C radicals as POBN/●C14H21O4(EIC of m/a 448, tR≈ 11.9, 14.8, and 23.3 min) were also generated. Indeed, all these fragmented free radical products, i.e., radicals generated from β-scission, were generated from the C-15 oxygenation of COX-catalyzed AA peroxidation and detected in the POBN spin-trapping experiment. A long-chain molecule of m/z 548 (EIC at tR≈ 26.2 min)corresponding to the POBN adduct of ●C20H34O5was also formed in the cell-PBS system due to the rearrangement from a PGF2-type alkoxyl radical to a carbon-centered radical(Scheme 1) [29].
Fig. 1.
LC/MS chromatogram (EICs) of POBN adducts formed from cellular COX-catalyzed AA and DGLA peroxidation. HCA-7 colony 29 cells were trypsinized, harvested, and suspended in PBS and treated with POBN and PUFAs as described in Materials and Methods. After a 30-min incubation, the reaction was stopped using ACN (1/1, v/v), and the supernatant was collected, centrifuged, and condensed for LC/MS analysis. (A) EICs of m/z 296, m/z 448, m/z 266 and m/z 548 as formation of POBN/●C5H11, POBN/●C6H13O, POBN/●C14H21O4 and POBN/●C20H34O5 from the C-15 oxygenation of COX-catalyzed AA peroxidation; (B) EICs of m/z 266 and m/z 296 from the formation of POBN/●C5H11, POBN/●C6H13O from C-15 oxygenation of COX-catalyzed DGLA peroxidation; and (C) EICs of m/z 324 and m/z 354 from the formation of POBN/●C7H13O2 and POBN/●C8H15O3 from C-8 oxygenation of COX-catalyzed DGLA peroxidation.
Due to the shared structural moiety (C8-C20) between AA and DGLA, C-15 oxygenation (Scheme 2A) also takes place in COX-catalyzed DGLA peroxidation. Thus when HCA-7 colony 29 cells were incubated with POBN and DGLA in PBS at 30 min, two free radicals were also formed and observed that are identical to AA metabolism (Figure 1B): them/z 266 ion as the POBN/●C5H11 molecule (tR≈ 24.0 min) from the β-scission of a PGH1-type alkoxyl radical, and two isomers of m/z 296 as POBN/●C6H13O (tR≈ 12.4 and 17.8 min) from the β’-scission of a PGH1-type alkoxyl radical (Scheme 2A). Consistent with the previous (cell-free) experiments [32], the formation of●C=C free radicals as the second type of radical product of β′-scission from 15-oxygenation of DGLA was again not detected in the cellular catalyzed DGLA peroxidation and spin-trapping experiment. Besides C-15 oxygenation, however, C-8 oxygenation (Scheme 2) also takes place in HCA-7 colony 29 cellular COX-catalyzed DGLA peroxidation. Two exclusive free radicals were thus generated and observed as POBN/●C8H15O3 with m/z 354 (two isomers, tR≈ 7.5 and 8.4 min) and POBN/●C7H13O2 with m/z 324 ion (tR≈ 9.1 min, Figure 1C) from the β-scission and β′-scission of same alkoxyl radical, respectively, as proposed in Scheme 2B (e.g. C-8 oxygenation of DGLA[32]).
Although our Figure 1 data demonstrated radical identification in cellular COX-catalyzed AA and DGLA peroxidation for the first time, its biological application was problematic for several reasons: (1) in order to observe the EIC peaks of POBN adducts as in Figure 1, peroxidation and spin-trapping experiments must be conducted with a large number of cells (>108/mL); (2) an excessive amount of POBN (≥ 50 mM) and PUFAs(≥500 µM) must also be introduced, even though such high doses of supplements could lead to cell death within 1 h; and (3) the cell culture media must be replaced by PBS for the incubation. Thus, only very short incubation times(~ 30 min)could be achieved for cellular peroxidation and spin-trapping. All these facts indeed restrict the biological application of the detection method in Figure 1, since the common parameters of cell growth, e.g., proliferation, apoptosis, and cell cycle distribution, must be assessed after a long incubation time.
Measurement of Hydroxylamines (i.e., Reduced POBN Spin Adducts) As Radical Products
In order to characterize AA- and DGLA-derived free radicals formed under normal cell growth conditions, we examined the reduced forms of the adducts. We proposed that due to the cellular reducing environment and the presence of reducing components in the culture media, the POBN radical adducts (ESR-active form) could be readily reduced to their ESR-silent redox forms, as hydroxylamines (Scheme 3). The hydroxylamine is a more stable radical product compared to the radical form (ESR-active) of the POBN adduct. It should be accumulated during normal cell incubation and thus be readily screened by LC/MS.
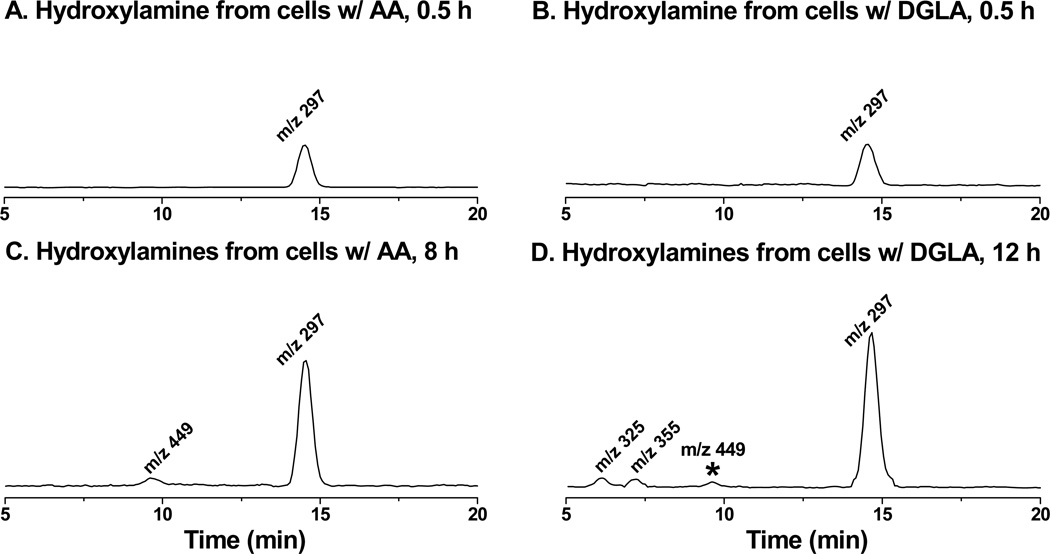
Indeed, even though condensing procedures and SPE extraction were applied, no ESR-active forms of POBN radical adducts could be measured by either LC/ESR or LC/MS (data not shown) when HCA-7 colony 29 cells were grown in cell culture media supplemented by 20 mM POBN and 100 µM AA or DGLA. However, the reduced product of the POBN/●C6H13O molecule, i.e., hydroxylamine with its corresponding m/z 297 ion, could clearly be projected at tR≈ 14.5 min in cellular COX-catalyzed AA peroxidation (Figure 2A). Unlike the two isomers of m/z 296 ions that could be generated from the cell free COX system [29, 32] and the cell-PBS system (Figure 1), only one isomer of m/z 297 was observed as the relevant hydroxylamine. In fact, this isomer is the major radical product formed in cellular COX-catalyzed AA peroxidation at all experimental points. The exclusive radical in COX-catalyzed AA peroxidation, seen as the reduced product(hydroxylamine) of POBN/●C14H21O4 for the m/z 449 EIC peak (tR≈ 9.6 min, Figure 2C), did not show up until the 8 h time point. However, reduced products of POBN/●C20H34O5 and POBN/●C5H11(Scheme 1) were not detected in cellular COX-catalyzed AA peroxidation at any experimental points.
Fig. 2.
LC/MS chromatogram (EICs) of hydroxylamines formed from cellular COX-catalyzed PUFA peroxidation. HCA-7 colony 29 cells were cultured in normal culture media supplemented with PUFAs and POBN as described in Materials and Methods. At the experimental time points, cell culture medium together with cells that were scrubbed and homogenized was mixed with a stop solution of ACN (1:1, v/v). The hydroxylamines were then extracted by SPE, condensed, and then subjected to LC/MS and LC/MS2 analysis as described in Materials and Methods. (A) EIC of m/z 297 ion as reduced POBN adduct of ●C6H13O formed from COX-catalyzed peroxidation in cells treated by AA at 30 min; (B) EIC of m/z 297 formed in COX-catalyzed peroxidation from cells treated by DGLA at 30 min; (C) EIC of m/z 297 and m/z 449 (reduced POBN adduct of ●C14H21O4) formed in COX-catalyzed peroxidation from cells treated by AA at 8 h; and (D) EIC of m/z 297, m/z 325 and m/z 355 (reduced POBN adducts of ●C7H13O3 and ●C8H15O3, respectively) formed in COX-catalyzed peroxidation from cells treated by DGLA at 12 h. Note, the asterisked m/z 449 ion in Fig 2D represents the reduced POBN adduct of ●C6H13O generated from COX-catalyzed AA peroxidation where DGLA is converted to AA by Δ-5 desaturase.
The same reduced product of POBN/●C6H13O as the EIC of m/z 297 (tR≈ 14.5 min) was also projected as the major hydroxylamine in COX-catalyzed DGLA peroxidation at the 0.5 h and 12 h time points (Figure 2B). The formation of the reduced products of POBN/●C7H13O2 and POBN/●C8H15O3 (i.e., two exclusive radical adducts) from COX-catalyzed DGLA peroxidation as EIC peaks of m/z 325and m/z 355 (tR≈ 6.1 and 7.1 min, Figure 2D) started to show up at 8 h and greatly accumulated after 12 h. Interestingly, although no ●C=C radical could form from DGLA, the hydroxylamine with an m/z 449 ion (asterisked peak in Figure 2D) as the reduced POBN adduct of ●C14H21O4 (e.g. exclusive ●C=C in COX-catalyzed AA peroxidation) also started to show up at 12 h. Formation of the exclusive AA-derived radicals from DGLA-mediated peroxidation indicated that a certain amount of AA was generated from DGLA by Δ-5 desaturase during incubation. This generation was confirmed by LC/MS analysis of PUFA profiles in later experiments.
Identification of Hydroxylamines As Radical Products via Dual Spin-Trapping and LC/MS2
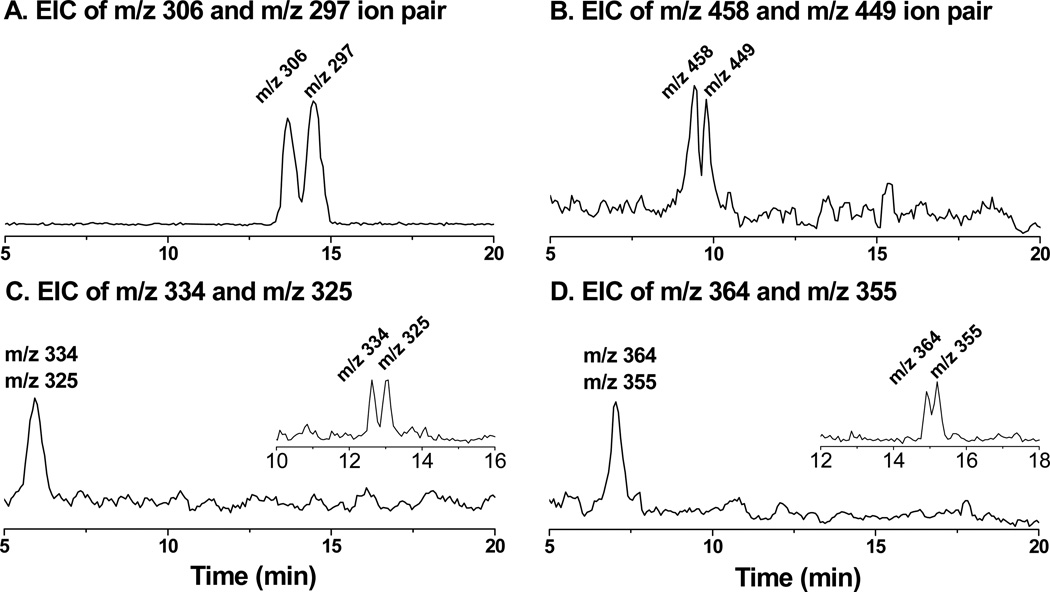
Dual spin-trapping experiment along with LC/MS detection [29–33] was used for recognition and also identification of a number of isomers of radical adducts and their structures. When a 50:50 mixture of POBN and d9-POBN together are applied in the cellular spin-trapping experiments, each hydroxylamine should be singled out in LC/MS as an ion pair with a 9-Da difference (Scheme 3).
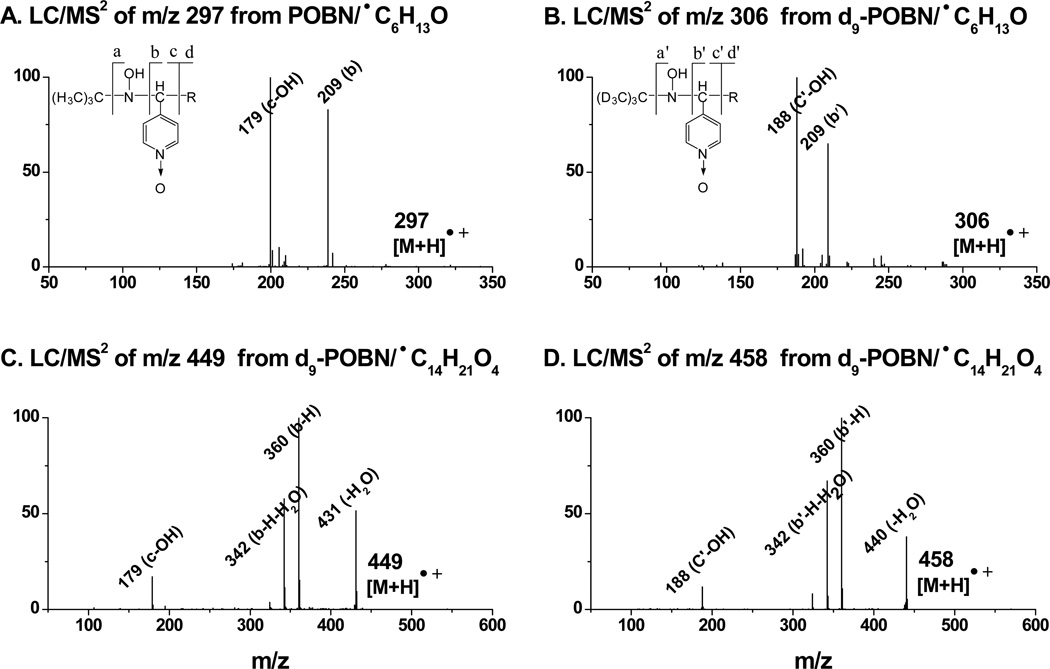
Indeed, detection of ion pairs of m/z 306 vs. m/z 297(EIC peaks at tR≈ 14.5 and 13.8 min, Figure 3A) corresponded to the formation of the reduced products (i.e., hydroxylamines) of d9/d0-POBN/●C6H13O. Similarly, detection of ion pairs of m/z 458 vs. m/z 449 (EIC peaks at tR≈ 9.6 and 9.3 min, Figure 3B) corresponded to the formation of the reduced products of d9/d0-POBN/●C14H21O4. One isomer for each of those hydroxylamines that could form in cellular COX-catalyzed AA peroxidation under normal culture conditions was validated by the dual spin tripping experiments. Fragmentation patterns from the LC/MS2 analysis (Figure 4) of m/z 297 vs. m/z 306 and m/z 449 vs. m/z 458 further confirmed the structural assignment of hydroxylamines, so it can be concluded that the relevant spin-trapped radicals, i.e.,●C6H13O and ●C14H21O4were in fact generated from cellular COX-catalyzed AA peroxidation.
Fig. 3.
LC/MS chromatogram (EICs) of d0/d9-hydroxylamines formed from cellular COX-catalyzed PUFA peroxidation and dual spin-trapping reaction. HCA-7 colony 29 cells were cultured under cell growth conditions for 12 h in the presence of d0/d9-POBN spin trap (50:50) as described above. (A) EIC of m/z 306 and m/z 297 as d9/d0-POBN adducts of ●C6H13O formed from cells treated with AA; (B) EIC of m/z 458 and m/z 449 as d9/d0-POBN adducts of ●C14H21O4 formed from cells treated with AA; (C) EIC of m/z 334 and m/z 325 as d9/d0-POBN adducts of ●C7H13O3 formed from cells treated with DGLA; and (D) EIC of m/z 364 and m/z 355 as d9/d0-POBN adducts of ●C8H15O3, formed from cells treated with DGLA. Note, the same EICs with different abundances of m/z 306 and m/z 297 as in Fig 3A were obtained from cells treated with DGLA (data not shown). A modified LC gradient condition in the insert in Figs. 3C-3D is: (i) 0 – 40 min, 0% to 60% B; (ii) 40 – 43 min, 60% to 95% B; and (iii) 43 – 50 min (isocratic), 5% A and 95% B.
Fig. 4.
LC/MS2, spectra of hydroxylamines formed from COX-catalyzed AA peroxidation. (A and B) LC/MS2 of m/z 297 and m/z 306 of do/d9-POBN adducts of ●C6H13O, respectively; and (C and D) LC/MS2 of m/z 449 and m/z 548 of do/d9-POBN adducts of ●C14H21O4, respectively. The fragmentation patterns of m/z 297 and m/z 449 in the POBN experiment have counterparts in the d9-POBN spin trapping experiment (e.g. a, b, c, and d ions vs. a′, b′, c′, and d′ ions).
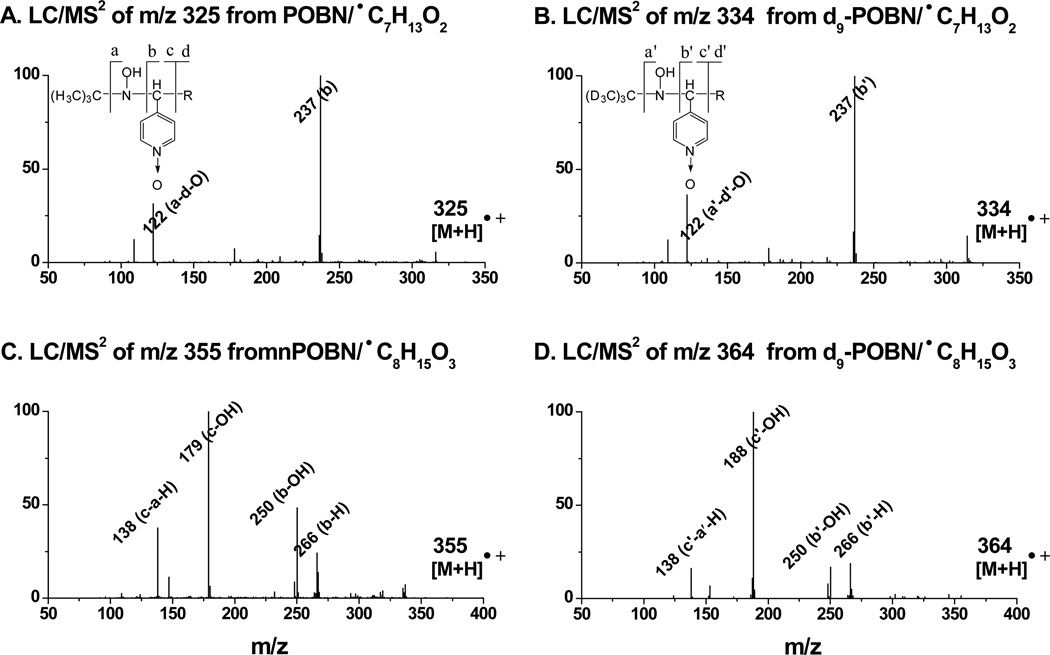
However, detection of the ion pairs of the reduced products of d9/d0-POBN/●C7H13O2 as m/z 334 vs. m/z 325 and d9/d0-POBN/●C8H15O3 as m/z 364 vs. m/z 355from COX-catalyzed DGLA peroxidation was not achieved under the current elution conditions. In fact, both m/z 325 and m/z 334 ions were eluted at tR≈5.96min (Figure 3C), while m/z 355 and m/z 364 ions were co-eluted at tR≈ 7.03 min (Figure 3D). The retention time (~6 – 7 min) appears too short to separate theseion pairs. When a modified gradient was applied, both ion pairs of m/z 334 vs. m/z 325 (tR≈ 12.6 and 13.0 min) and m/z 364 vs. m/z 355 (tR≈ 14.9 and 15.2 min) could be measured (insets of Figures 3C-3D). Again, LC/MS2analysis of m/z 325 vs. m/z 334 and m/z 355vs. m/z 364 ions (Figure 5) allowed us to further confirm the structural assignment of hydroxylamines, and we conclude that the relevant radicals (e.g. ●C7H13O2 and ●C8H15O3) were indeed generated from cellular COX-catalyzed DGLA peroxidation.
Fig. 5.
LC/MS2 spectra of hydroxylamines formed from COX-catalyzed DGLA peroxidation. (A and B) LC/MS2 of m/z 325 and m/z 334 of do/d9-POBN adducts of ●C7H13O3, respectively; and (C and D) LC/MS2 of m/z 355 and m/z 364 of do/d9-POBN adducts of ●C8H15O3, respectively. The fragmentation patterns of m/z 325 and m/z 355 in the POBN experiment have counterparts in the d9-POBN spin trapping experiment (e.g. a, b, c, and d ions vs. a′, b′, c′, and d′ ions).
Profiles of DGLA/AA, PGEs, and Hydroxylamines
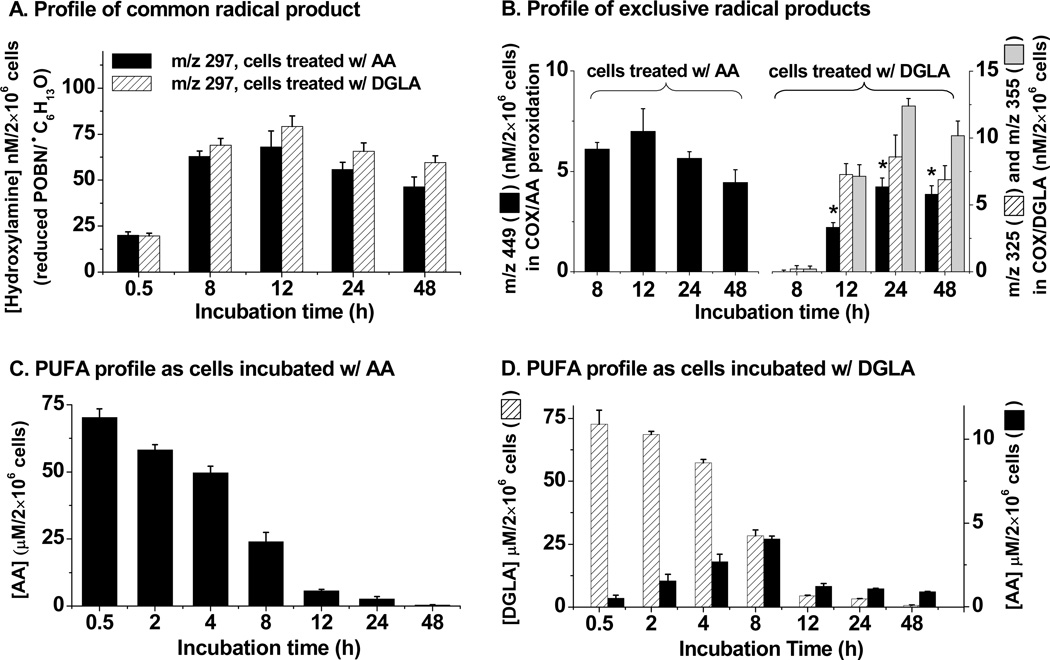
The formation of radicals (●C6H13O, Schemes 1 – 2)from cellular COX-catalyzed AA as well as DGLA peroxidation was monitored by detection of hydroxylamine as the m/z 297 ion. In both systems, the hydroxylamine of POBN/●C6H13O was found to continually increase and reach a peakat 12 h, and to then slightly decrease over two days (<15%, Figure 6A). In addition, a similar amount of m/z 297 was only measured in both systems at~ 0.5 h, while on average ~ 10% more of this hydroxylamine was observed in cellular COX-catalyzed peroxidation from DGLA-treatment vs. AA-treatment for up to 48 h.
Fig. 6.
Time course of hydroxylamines, AA, and DGLA in cellular (HCA-7 colony 29) COX-catalyzed peroxidation. (A) Profile of the radical product (m/z 297) formed in common from both COX-catalyzed AA and DGLA peroxidation in cells treated with AA and DGLA; (B) Profile of exclusive radical products in COX/AA and COX/DGLA peroxidation, respectively. The exclusive radical product in COX-catalyzed AA peroxidation was a hydroxylamine of m/z 449, while the exclusive radical products in COX-catalyzed DGLA peroxidation were hydroxylamines of m/z 325 and m/z 355. Asterisked m/z 449 was the hydroxylamine generated from COX-catalyzed peroxidation of AA, which was converted from DGLA; (C) Profile of AA from cell culture medium with cells treated by 100 µM AA; no DGLA could be measured; and (D) Profile of DGLA and AA in cell culture medium with cells treated by 100 µM DGLA. Hydroxylamines were quantified by d9-POBN, while AA and DGLA were quantified by internal standard AA-d8 and DGLA-d6 as described in Materials and Methods, respectively. The amounts of PUFAs and hydroxylamines formed in the cellular peroxidation reaction were also normalized to the numbers of live cells at different incubation times. Data were expressed as means ± SD from n≥3.
The exclusive radical product of POBN/●C14H21O4 formed from COX-catalyzed AA peroxidation, as monitored by the detection of hydroxylamine of m/z 449, was not detected until the 8 h time point, and reached a peak at 12 h as well (Figure 6B). Two exclusive radical products of POBN/●C7H13O2 and POBN/●C8H15O3 in COX-catalyzed DGLA peroxidation, represented by the detection of two hydroxylamines as m/z 325 and m/z 355, respectively, also showed up at the 8 h time point, had greatly accumulated at 12 h, and remained at a similar level for up to 48 h (Figure 6B). Interestingly, the hydroxylamine of m/z 449 (the exclusive radical product of COX-catalyzed AA peroxidation) could also be measured at 12 h (asterisked in Figure 6B) with an amount similar to the two exclusive radical products of COX-catalyzed DGLA peroxidation, i.e., hydroxylamines of m/z 325 and m/z 355, although no AA was added into the culture media. The detection of an exclusively AA-derived radical from cellular peroxidation with DGLA treatment suggested that DGLA could be converted to AA (by Δ-5 desaturase) and that both PUFAs were competitively metabolized by COX. When cells were incubated with DGLA, the profile of PUFAs in cellular COX peroxidation was consistent with the proposal that AA is continually converted from DGLA (Figure 6D); meanwhile, both AA and DGLA were competitively peroxidized by COX to form PGEs (Table 1). When cells were co-treated with DGLA and CP-24879, not only was the AA converted from DGLA very limited, but also the hydroxylamine of m/z 449 formed, representing the exclusive radical product formed in COX-catalyzed AA peroxidation, was barely detected at 24 h and slightly increased at 48 h (data not shown).
Table 1.
Formation of PGEs in cellular COX peroxidation and cell growth response. Cell cycle analysis (PI staining) and cell proliferation assays (MTS) of HCA-7 colony 29 cells treated with AA, DGLA and DGLA in combination with CP-24879.
| Cells (HCA-7 Colony 29) Cultured w/ |
Formation of PGs (nM/2×106 cells) | Cell Cycle Distribution | Cell Proliferation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [PGE1] | [PGE2] | % in G1 Phase | % Viability | |||||||
| 8 h | 24 h | 48 h | 8 h | 24 h | 48 h | 8 h | 24 h | 48 h | 48 h | |
| Controla | N.D.b | 0.9±0.2 | 0.6±0.4 | N.D. | 36.6±1.6 | 40.3±3.8 | 44.6±2.2 | 100 | ||
| AA (100 µM) |
N.D. | 101.5±5.3 | 80.6±6.0 | 68.6±7.9 | 40.4±7.2 | 41.6±3.8 | 43.7±3.2 | 112.1±3.9 | ||
| DGLA (100 µM) |
81.3±0.3 | 70.6±2.2 | 48.1±1.9 | 19.3±0.3 | 32.6±1.7 | 48.6±0.5 | 46.3c±7.6 | 42.7±2.3 | 38.3±1.2 | 109.1±5.7 |
| DGLA w/ 5.0 µM CP |
92.3±4.2 | 76.8±8.3 | 64.0±6.3 | 6.8±1.3 | 9.3±3.9 | 14.4±5.4 | 44.5c±5.2 | 47.0c±3.4 | 45.3±2.6 | 98.3d±5.0 |
% cell viability was compared with control group. Control cells were incubated with 0.1 % dimethylsulfoxide and 0.1 % ethanol (final concentration);
no detected or data represent the mean ± SD derived from three separate experiments with triplicate wells per condition;
P < 0.05, significant G1 delay vs. corresponding controls; and
significantly different vs. treatment of AA and DGLA. In addition, G2/M phase delays were also observed from 8 h treatments of DGLA and 8 h-24 h treatment of DGLA with CP24879 (data not shown).
Cell Growth Response from Treatments of PUFAs, PGEs, and Free Radical-Derivatives
The use of the hydroxylamine to monitor the formation of free radicals in COX-catalyzed peroxidation allowed us to examine the association of free radicals with the cell growth response. The possible relationships among of cell growth vs. PUFAs and their COX-catalyzed metabolites, including PGEs and the derivatives of free radicals, were assessed (Tables 1–3). After 8 h incubation, treatments of DGLA (with/without CP-24879)were associated with sustained G1 and G2/M phase arrest vs. control (Table 1). However, almost no difference of cell distribution was found after one-day treatment between AA and DGLA, while a one-day treatment of DGLA along with CP-24879 still resulted in sustained G1 and G2/M phase arrest. In addition, the combination treatment inhibited cell proliferation to the level of the control, in contrast to the treatments of AA or DGLA alone (Table 1).
Table 3.
Cell cycle distribution from 48 h treatment of free radical derivatives
| [Product] (µM) |
Cell Cycle Distribution at 48 h from the Treatment of |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1-Hexanol | Heptanoic Acid | 8-OH-Octanoic Acid | |||||||
| G1 | S | G2/M | G1 | S | G2/M | G1 | S | G2/M | |
| 0 (control) | 44.6±2.2 | 32.7±1.0 | 22.7±0.3 | 44.6±2.2 | 32.7±1.0 | 22.7±0.3 | 44.6±2.2 | 32.7±1.0 | 22.7±0.3 |
| 0.01 | 44.5±1.0 | 32.8±2.4 | 22.7±3.1 | 45.3±0.2 | 35.1±1.9 | 19.6±2.9 | 54.8a±0.3 | 26.4±0.8 | 18.8±1.4 |
| 0.1 | 49.0±1.6 | 31.0±1.2 | 20.0±1.6 | 44.7±1.2 | 30.1±3.0 | 25.2±2.4 | 51.4a±0.3 | 26.8±0.4 | 21.8±0.7 |
| 1.0 | 45.9±2.5 | 32.5±3.8 | 21.6±1.9 | 48.5±3.9 | 29.8±5.3 | 21.7±3.6 | 50.1a±3.4 | 28.0±4.2 | 21.9±2.9 |
A range of 0.01 to 1.0 µM hexanol (derived from ●C6H13O), heptanoic acid (derived from ●C8H15O3), and 8-hydroxyoctanoid acid (derived from ●C7H13O2) were used to treat cells, which were then tested for cell cycle distribution (PI staining). Data represent the mean ± SD derived from three separate experiments with triplicate wells per condition.
at 48 h, treatment of 8-OH-octanoic acid resulted in significant G1 phase delay compared with the control group (cells were incubated with 0.1 % ethanol, P < 0.05). In addition, both G1 and G2/M phase delays could be observed from the treatment time points (e.g. 8 h and 12 h) of 8-OH-octanoic acid and hexanol (data not shown). Note that neither PGE1 nor PGE2 treatments resulted in any G1 or G2/M arrest at 48 h (data not shown).
In order to assess more directly the association of free radicals with cell growth, three radical derivatives, 1-hexanol, heptanoic acid, and 8-hydroxyoctanoic acid (referring to ●C6H13O, ●C7H13O2and ●C8H15O3respectively) were used to treat cells in order to test their effects on cell proliferation. Although much higher concentrations of PGEs (~40 to 4,000 fold vs. the PGEs per number of cells that were actually quantified in our AA- and DGLA-treatments, Table 1) were directly added to culture media, neither PGE1 nor PGE2 had any inhibitory effect on cell proliferation (Table 2). However, at similar concentrations, 8-hydroxyoctanoic acid (a derivative of ●C8H15O3, which is an exclusive product from COX catalyzed DGLA peroxidation) could inhibit cell proliferation (Table 2). Interestingly, the treatment with 1-hexanol, a derivative of ●C6H13O, which is the major product from both AA and DGLA peroxidation, also resulted in inhibition of cell proliferation, while another exclusive radical (●C7H13O2) derivative from COX-catalyzed DGLA peroxidation, heptanoic acid, did not affect cell proliferation. Our cell distribution studies showed that the treatment with 8-OH-octanic acid (an exclusive DGLA-derived radical metabolite) causes sustained G1 arrest at all tested time points, including the 48 h point (Table 3). In addition, G2/M delay could be observed from the treatment with 8-OH-octanic acid from 8 h to 12 h (data not shown). On the other hand, the common product (1-hexanol) only resulted in G1 and G2/M arrest at the short time points, typically 8 h (data not shown), and no cell cycle delay was detected at 48 h (Table 3).
Table 2.
Effect on cell proliferation from PGEs and free radical derivatives
| [Product] (µM) |
Cell Proliferation from the Treatment (% Viability at 48 h vs. Controla) | ||||
|---|---|---|---|---|---|
| PGE1 | PGE2 | 1-Hexanol | Heptanoic Acid | 8-OH-Octanoic Acid | |
| 0.01 | 108.6±3.3 | 108.5±4.3 | 89.8b±2.1 | 98.6±2.1 | 77.1c±5.1 |
| 0.1 | 109.5±2.7 | 112.7±2.9 | 87.7b±2.5 | 97.4±4.0 | 76.7c±5.3 |
| 1.0 | 106.2±3.5 | 116.9±5.4 | 90.2b±4.6 | 103.5±5.3 | 82.3c±6.2 |
At the range of 0.01 to 1.0 µM, PGE1, PGE2, 1-hexanol (derived from ●C6H13O), heptanoic acid (derived from ●C8H15O3), and 8-hydroxyoctanoid acid (derived from ●C7H13O2) were used to treat cells and tested for cell proliferation assay (MTS). Data represent the mean ± SD derived from three separate experiments with triplicate wells per condition.
% cell viability was compared with the control group (cells were incubated with 0.1 % ethanol);
significantly different (P < 0.01) vs. control; and
significantly different (P < 0.005) vs. control.
DISCUSSION
In order to investigate the association between cancer cell growth and free radicals generated from COX-catalyzed AA vs. DGLA peroxidation, we have for the first time characterized free radicals formed from HCA-7 colony 29 cells under normal growing conditions. Unlike the cell-PBS system (Figure 1) in which large doses of POBN and PUFAs must be introduced to generate enough free radicals to be trapped and measured, our refined spin-trapping experiment grew cells in a normal culture medium with a much lower concentration of POBN and PUFAs. Then, instead of measuring ESR-active radical adducts, we actually measured a reduced form (hydroxylamines, Scheme 3) of the POBN adducts using LC/MS and LC/MS2 since hydroxylamines are much more stable redox forms of spin adducts than ESR-active POBN adducts, especially in cell culture media.
Due to their longer life times in cell culture media, hydroxylamines accumulated during the incubation if POBN was accessible. We hypothesized that a single dose of POBN (~ 20 mM) in culture medium would continuously trap the free radicals generated from long term incubation, i.e., day-long cellular COX-catalyzed AA and DGLA peroxidation. Thus, we tested the radical trapping ability of POBN in cell culture medium to see how effectively a single dose of 20 mM POBN can trap free radicals during long time incubation. At different experimental time points, 20 mM POBN (dissolved and incubated in cell-free culture media) was mixed with ethanol and 1.0 mM Fe2+ (to oxidize 0.2 mM ethanol with O2 to form hydroxyethyl radicals), generating a six-line spectrum of POBN/●C2H5O (aN ≈ 15.7 G and aH ≈ 2.8 G) in ESR (data not shown). The signal intensities of the ESR spectra of POBN/●C2H5O were used to compare and calculate the radical trapping ability of POBN at different time points (from 0.5 h to 48 h). Although it dropped ~ 30% in the first 2-4 h of incubation, about 60% of POBN’s radical trapping ability still remained after a two-day incubation. Meanwhile, metabolism or loss of POBN (20 mM) incubated in the culture media was negligible up to 48 h (via LC/MS analysis, data not shown). Our results suggested that a single dose of 20 mM POBN in culture media can consistently and effectively trap free radicals during up to two days of cellular COX-catalyzed peroxidation.
In both COX-catalyzed DGLA and AA systems, 8 h and 12 h incubation seem to be the critical time points for free radical formation. The overall radical production in both peroxidation systems accumulated and approached a peak at about 12 h (Figures 6A-6B). Considering POBN’s decreased trapping ability in culture media, in reality the radical production would be at a plateau after 12 h. The exclusive radical products, i.e., the m/z 449 hydroxylamine as the reduced product of POBN/●C14H21O4, and the m/z 355 and m/z 325 hydroxylamines as reduced products of POBN/●C8H15O3 and POBN/●C7H13O2, generated from COX-catalyzed AA and DGLA, respectively, started to show up at 8 h. The appearance of the hydroxylamine of m/z 449 (referring to ●C14H21O4) was much delayed (with much less produced as well) compared to the hydroxylamine of m/z 297 (referring to ●C6H13O). These two free radicals were expected to form at the same time points and in similar quantities since they are two co-metabolites fragmenting from the same product through β’-scission of PGF2-alkoxyl radicals as proposed elsewhere (Scheme 1 [29]). The delayed appearance of m/z 449 suggested that β’-scission could also occur on alkoxyl radicals derived from other types of PGs (PGH2 etc.) during COX-catalyzed AA peroxidation, instead of taking place at the PGF2 stage [29] in which the ●C=C radical, whose adduct was reduced to the m/z 449 hydroxylamine, was formed at the same time as the hydroxylamine of m/z 297 (reduced POBN adduct of ●C6H13O). Again, β’-scission of a PGH1-type alkoxyl radical did not result in a ●C=C radical from DGLA (Scheme 2 [32]) that could form ●C14H21O4 as in AA peroxidation. In fact, hexanol (a ●C6H13O derivative) was detected in COX/AA and COX/DGLA systems by GC/MS (data not shown) from cellular experiments in which POBN was absent.
In addition, the formation of a similar amount of hydroxylamine of m/z 449 (asterisked in Figure 6B) as the hydroxylamines of m/z 355 and m/z 325 during COX-catalyzed peroxidation in cells treated by DGLA (12 h to 48 h) suggested that once formed (converted from DGLA by Δ-5 desaturase), AA is a more favored substrate than DGLA for COX peroxidation. The formation peak of AA (from DGLA) was found at 8 h although the concentration of DGLA was still much higher than AA (~5-6 fold, Figure 6D). DGLA could be very effectively converted to AA; in turn both fatty acids competitively compete with each other for COX peroxidation. When cells were incubated with AA, neither DGLA nor DGLA exclusive hydroxylamines of m/z 355 and m/z 325 were detected. However, a considerable amount of m/z 449 was detected as early as 8 h (Figure 6B).
The formation of the hydroxylamine of m/z 449 was generally associated with cell growth promotion (Table 1). The sustained G1 and G2/M phase cell cycle arrests were observed at the time points and the treatments in which the hydroxylamine of m/z 449 was barely measurable. For example, treatment of DGLA ~ 8 h was correlated with sustained G1(Table 1) and G2/M (data not shown)phase arrest, while the combined treatment with DGLA and CP-23978 was correlated with sustained G1 and G2/M phase cell cycle arrest within one day. The considerable abundance of the hydroxylamine of m/z 449 formed in cells after treatment with AA (8 h to 24 h, Figure 6A) and the one-day DGLA treatment was correlated with a lack of any difference in G1 and G2/M phase cell cycle distribution vs. the control.
The two series of prostaglandins, PGs1 and PGs2, are well-known bioactive metabolites. Unlike PGs2s, which are generally viewed as pro-inflammatory and pro-carcinogenic PGs, PGs1 may possess anti-inflammatory and anti-cancer activity. However, some research studies of PGs on cell growth were considered somewhat unrealistic since much higher concentrations of PGs (particularly PGE1) were used, compared to the concentrations that could form under normal cell culture conditions with fatty acid supplementation. It was reported that no more than 13 nM of PGEs could form from cultured cells without a fatty acid supplement [35–38]. The colon cancer cell (HT-29) growth could be stimulated up to 45% by PGE2 at a range of 0.5 µM to 10 µM [39]. At a similar concentration, PGE2 could also induce the expression of vascular endothelial growth factor, one of the major regulators for LS-174T cell angiogenesis, thus exerting pro-oncogenic actions in colorectal carcinogenesis[40]. On the other hand, a dose of PGE1 (30 µg/mL) was reported to inhibit the proliferation of HeLa cells [24] and B16-F10 cells [23]. However, at lower doses (3.0 µg/mL), PGE1 could not inhibit cell proliferation, and even increased cell growth [24].
In our experiments, no more than 0.12 µM of PGEs (from experiment of 2×106 cells, Table 1) could be detected from the 100 µM PUFA treatments. When a range of 0.01 µM to 1.0 µM PGE (~ 40 to 4000 fold higher than the PGEs per number of cells actually formed and measured in our experiments, Table 1) was used to directly treat an HCA-7 colony with 29 cells in our study, neither PGE1 nor PGE2 showed much effect on cell proliferation (Table 2) and cell cycle distribution (data not shown). The inconsistent cell growth responses of PGEs from the PUFA treatment (Table 1) and the PGEs used to directly treat cells (Tables 1–3) suggested that rather than PGEs, free radicals and derivatives should be considered for their possible bioactivities. Interestingly, inhibited cell proliferation effects were observed from some of the tested free radical derivatives at the same concentrations as the PGEs in Tables 2–3. The 8-hydroxyoctanoic acid (a derivative of ●C8H15O3 as an exclusive product from 8-C oxygenation in COX catalyzed DGLA peroxidation) showed some anti-proliferation effects and resulted in G1 phase delay (G2/M delay at a short time period as well), while another exclusive radical (●C7H13O2) derivative from 8-C oxygenation of DGLA (heptanoic acid) did not affect cell proliferation or cell cycle distribution. However, as the common and major product from 15-C oxygenation of both AA and DGLA, 1-hexanol (a derivative of ●C6H13O) was also correlated with inhibition of cell proliferation and G1 and G2/M delay at time points of 8 h and 12 h (data not shown), although not with different cell cycle distribution at 48 h vs. control (Table 3).
Although no mechanism has so far been defined for the tested exclusive free radical derivatives, our results suggested that the radical metabolites derived from C-8 oxygenation of DGLA had an inhibitory effect on colon cancer cell growth. In addition, based on the time course of ●C=Cradical generation and the related cell growth response (Figure 6 and Table 1), it is logical to propose that ●C14H21O4 or its derivative corresponds with AA-promoted cell growth. In our future studies, compounds with similar structure of ●C14H21O4 free radical and its derivatives will be screened or synthesized, and used for testing the effects on cell growth. In conclusion, the data in this study indicate that the novel free radicals as well as the related radical derivatives formed from COX peroxidation should be the new targets to be tested for their promoting or inhibiting effect on cancer cell growth. Understanding these radicals and their related radical reactions may allow us to advance our knowledge of the mechanisms of PUFA’s bioactivity in cancer biology.
Pathways and products of COX-AA/-DGLA peroxidation were confirmed in cell model.
Radicals were characterized and quantified under normal cell culture conditions.
Association between free radicals and cell growth response was assessed.
Approach allows other research labs to conduct radical study in similar cell model.
ACKNOWLEDGMENTS
This work was supported by NIH Grant 1R15CA140833 and P20 RR015566. Authors would like to thank Dr. YH Yang (a scientist in the Synthesis Facility, Center for Protease Research, NDSU) for help with the d9-POBN synthesis, and Dr. T Wang (a scientist in the Core Biology Facility, NDSU) for cell cycle distribution analysis. The authors would also like to thank Drs. E Wu and B Guo for their helpful discussions on our cellular biology experiments and results.
LIST OF ABBREVIATION
- AA
arachidonic acid
- ACN
acetonitrile
- COX
cyclooxygenase
- DGLA
dihomo-gamma linoleic acid
- EIC
extracted ion chromatogram
- ESR
electron spin resonance
- HOAc
glacial acetic acid
- HPLC
high performance liquid chromatography
- MRM
multiple reaction monitoring
- MS
mass spectrometry
- POBN
α-[4-pyridyl 1-oxide]-N-tert-butyl nitrone
- PUFAs
polyunsaturated fatty acids
- TIC
total ion chromatogram
- tR
retention time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 2.Smith WL. Nutritionally essential fatty acids and biologically indispensable cyclooxygenases. Trends Biochem. Sci. 2008;33:27–37. doi: 10.1016/j.tibs.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–8873. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Xu X, He P. Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol. Med. Report. 2011;4:25–29. doi: 10.3892/mmr.2010.379. [DOI] [PubMed] [Google Scholar]
- 6.Thiel A, Narko K, Heinonen M, Hemmes A, Tomasetto C, Rio MC, Haglund C, Makela TP, Ristimaki A. Inhibition of cyclooxygenase-2 causes regression of gastric adenomas in trefoil factor 1 deficient mice. Int. J. Cancer. 2012;131:1032–1041. doi: 10.1002/ijc.27331. [DOI] [PubMed] [Google Scholar]
- 7.Hammes LS, Tekmal RR, Naud P, Edelweiss MI, Kirma N, Valente PT, Syrjanen KJ, Cunha-Filho JS. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecol. Oncol. 2008;110:445–451. doi: 10.1016/j.ygyno.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 8.Roberts HR, Smartt HJ, Greenhough A, Moore AE, Williams AC, Paraskeva C. Colon tumour cells increase PGE2 by regulating COX-2 and 15-PGDH to promote survival during the microenvironmental stress of glucose deprivation. Carcinogenesis. 2011;32:1741–1747. doi: 10.1093/carcin/bgr210. [DOI] [PubMed] [Google Scholar]
- 9.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Upregulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 10.Sinicrope FA, Lemoine M, Xi L, Lynch PM, Cleary KR, Shen Y, Frazier ML. Reduced expression of cyclooxygenase 2 proteins in hereditary nonpolyposis colorectal cancers relative to sporadic cancers. Gastroenterology. 1999;117:350–358. doi: 10.1053/gast.1999.0029900350. [DOI] [PubMed] [Google Scholar]
- 11.Thiebaut AC, Chajes V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, Berrino F, Riboli E, Benichou J, Clavel-Chapelon F. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int. J. Cancer. 2009;124:924–931. doi: 10.1002/ijc.23980. [DOI] [PubMed] [Google Scholar]
- 12.Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, Gerber L, Taylor LA, McKeever MG, Freedland SJ. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr. Res. 2011;31:1–8. doi: 10.1016/j.nutres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Brown MD, Hart C, Gazi E, Gardner P, Lockyer N, Clarke N. Influence of omega-6 PUFA arachidonic acid and bone marrow adipocytes on metastatic spread from prostate cancer. Br. J. Cancer. 2010;102:403–413. doi: 10.1038/sj.bjc.6605481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case-control study. Int. J. Cancer. 2008;123:1974–1977. doi: 10.1002/ijc.23729. [DOI] [PubMed] [Google Scholar]
- 15.Sauer LA, Blask DE, Dauchy RT. Dietary factors and growth and metabolism in experimental tumors. J. Nutr. Biochem. 2007;18:637–649. doi: 10.1016/j.jnutbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Kimura Y, Kono S, Toyomura K, Nagano J, Mizoue T, Moore MA, Mibu R, Tanaka M, Kakeji Y, Maehara Y, Okamura T, Ikejiri K, Futami K, Yasunami Y, Maekawa T, Takenaka K, Ichimiya H, Imaizumi N. Meat, fish and fat intake in relation to subsite-specific risk of colorectal cancer: The Fukuoka Colorectal Cancer Study. Cancer Sci. 2007;98:590–597. doi: 10.1111/j.1349-7006.2007.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pozzi A, Yan XX, Macias-Perez I, Wei SZ, Hata AN, Breyer RM, Morrow JD, Capdevila JH. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J. Biol. Chem. 2004;279:29797–29804. doi: 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- 18.Leone V, di Palma A, Ricchi P, Acquaviva F, Giannouli M, Di Prisco AM, Iuliano F, Acquaviva AM. PGE2 inhibits apoptosis in human adenocarcinoma Caco-2 cell line through Ras-PI3K association and cAMP-dependent kinase A activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G673–G681. doi: 10.1152/ajpgi.00584.2006. [DOI] [PubMed] [Google Scholar]
- 19.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 20.Fan YY, Ramos KS, Chapkin RS. Cell cycle related inhibition of mouse vascular smooth muscle cell proliferation by prostaglandin E1: relationship between prostaglandin E1 and intracellular cAMP levels. Prostaglandins, Leukot. Essent. Fatty Acids. 1996;54:101–107. doi: 10.1016/s0952-3278(96)90066-6. [DOI] [PubMed] [Google Scholar]
- 21.Gianetti J, De Caterina M, De Cristofaro T, Ungaro B, Guercio RD, De Caterina R. Intravenous prostaglandin E1 reduces soluble vascular cell adhesion molecule-1 in peripheral arterial obstructive disease. Am. Heart J. 2001;142:733–739. doi: 10.1067/mhj.2001.118109. [DOI] [PubMed] [Google Scholar]
- 22.Takai S, Jin D, Kawashima H, Kimura M, Shiraishi-Tateishi A, Tanaka T, Kakutani S, Tanaka K, Kiso Y, Miyazaki M. Anti-atherosclerotic effects of dihomo-γ-linolenic acid in ApoE-deficient mice. J. Atheroscler. Thromb. 2009;16:480–489. doi: 10.5551/jat.no430. [DOI] [PubMed] [Google Scholar]
- 23.Fang W, Li H, Zhou L, Su L, Liang Y, Mu Y. Effect of prostaglandin E1 on TNF-induced vascular inflammation in human umbilical vein endothelial cells. Can. J. Physiol. Pharmacol. 2010;88:576–583. doi: 10.1139/y10-028. [DOI] [PubMed] [Google Scholar]
- 24.Tabolacci C, Lentini A, Provenzano B, Gismondi A, Rossi S, Beninati S. Similar antineoplastic effects of nimesulide, a selective COX-2 inhibitor, and prostaglandin E1 on B16-F10 murine melanoma cells. Melanoma Res. 2010;20:273–279. doi: 10.1097/CMR.0b013e328339d8ac. [DOI] [PubMed] [Google Scholar]
- 25.Sagar PS, Das UN. Cytotoxic action of cis-unsaturated fatty acids on human cervical carcinoma (HeLa) cells in vitro. Prostaglandins, Leukot. Essent. Fatty Acids. 1995;53:287–299. doi: 10.1016/0952-3278(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 26.Rouzer CA, Marnett LJ. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 2003;103:2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 27.Qian SY, Yue GH, Tomer KB, Mason RP. Identification of all classes of spin-trapped carbon-centered radicals in soybean lipoxygenase-dependent lipid peroxidations of ω-6 polyunsaturated fatty acids via LC/ESR, LC/MS, and tandem MS. Free Radic. Biol. Med. 2003;34:1017–1028. doi: 10.1016/s0891-5849(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 28.Qian SY, Guo Q, Mason RP. Identification of spin trapped carbon-centered radicals in soybean lipoxygenase-dependent peroxidations of ω-3 polyunsaturated fatty acids by LC/ESR, LC/MS, and tandem MS. Free Radic. Biol. Med. 2003;35:33–44. doi: 10.1016/s0891-5849(03)00217-x. [DOI] [PubMed] [Google Scholar]
- 29.Yu QF, Purwaha P, Ni KY, Sun CW, Mallik S, Qian SY. Characterization of novel radicals from COX-catalyzed arachidonic acid peroxidation. Free Radic. Biol. Med. 2009;47:568–576. doi: 10.1016/j.freeradbiomed.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Q, Shan Z, Ni K, Qian SY. LC/ESR/MS study of spin trapped carbon-centred radicals formed from in vitro lipoxygenase-catalysed peroxidation of γ-linolenic acid. Free Radic. Res. 2008;42:442–455. doi: 10.1080/10715760802085344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shan Z, Yu QF, Purwaha P, Guo B, Qian SY. A combination study of spin-trapping, LC/ESR and LC/MS on carbon-centred radicals formed from lipoxygenase-catalysed peroxidation of eicosapentaenoic acid. Free Radic. Res. 2009;43:13–27. doi: 10.1080/10715760802567606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao Y, Gu Y, Purwaha P, Ni KY, Law B, Mallik S, Qian SY. Characterization of free radicals formed from COX-catalyzed DGLA peroxidation. Free Radic. Biol. Med. 2011;50:1163–1170. doi: 10.1016/j.freeradbiomed.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purwaha P, Gu Y, Kelavkar U, Kang JX, Law B, Wu EX, Qian SY. LC/ESR/MS study of pH-dependent radical generation from 15-LOX-catalyzed DPA peroxidation. Free Radic. Biol. Med. 2011;51:1461–1470. doi: 10.1016/j.freeradbiomed.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian SY, Buettner GR. Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: An electron paramagnetic resonance spin trapping study. Free Radic. Biol. Med. 1999;26:1447–1456. doi: 10.1016/s0891-5849(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 35.Moore AE, Greenhough A, Roberts HR, Hicks DJ, Patsos HA, Williams AC, Paraskeva C. HGF/Met signalling promotes PGE2 biogenesis via regulation of COX-2 and 15-PGDH expression in colorectal cancer cells. Carcinogenesis. 2009;30:1796–1804. doi: 10.1093/carcin/bgp183. [DOI] [PubMed] [Google Scholar]
- 36.Tavolari S, Bonafe M, Marini M, Ferreri C, Bartolini G, Brighenti E, Manara S, Tomasi V, Laufer S, Guarnieri T. Licofelone, a dual COX/5-LOX inhibitor, induces apoptosis in HCA-7 colon cancer cells through the mitochondrial pathway independently from its ability to affect the arachidonic acid cascade. Carcinogenesis. 2008;29:371–380. doi: 10.1093/carcin/bgm265. [DOI] [PubMed] [Google Scholar]
- 37.Moonen HJJ, Dommels YEM, van Zwam M, van Herwijnen MHM, Kleinjans JCS, Alink GM, de Kok TMCM. Effects of polyunsaturated fatty acids on prostaglandin synthesis and cyclooxygenase-mediated DNA adduct formation by heterocyclic aromatic amines in human adenocarcinoma colon cells. Mol. Carcinog. 2004;40:180–188. doi: 10.1002/mc.20032. [DOI] [PubMed] [Google Scholar]
- 38.Dommels YE, Haring MM, Keestra NG, Alink GM, van Bladeren PJ, van Ommen B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE2 synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis. 2003;24:385–392. doi: 10.1093/carcin/24.3.385. [DOI] [PubMed] [Google Scholar]
- 39.Chell SD, Witherden IR, Dobson RR, Moorghen M, Herman AA, Qualtrough D, Williams AC, Paraskeva C. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Research. 2006;66:3106–3113. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- 40.Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 Stimulates the β-catenin/T cell factor-dependent transcription in colon cancer. J. Biol. Chem. 2005;280:26565–26572. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]