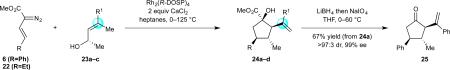
Table 3.
a Variations in Vinylic Substitution.
| |||||
|---|---|---|---|---|---|
| entry | comp'd | product | yield, %b | drc | ee, %d |
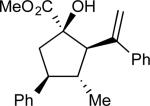
| 1 | a |
|
87 | >97:3 | 99 |
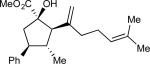
| 2 | b |
|
85 | >97:3 | 99 |
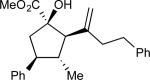
| 3 | c |
|
86 | >97:3 | 99 |
| 4 | d |
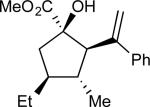
|
89 | >97:3 | 99 |
Reaction conducted with 23 (0.50 mmol, 1.0 equiv), 6 (0.60 mmol, 1.2 equiv) or 22 (1.0 mmol, 2.0 equiv) and Rh2(R-DOSP)4 (0.0005 mmol, 0.1 mol %), unless otherwise indicated.
Isolated yield of the major diastereomer.
Determined by 1H NMR analysis of the crude reaction residue.
Determined by HPLC analysis on a chiral stationary phase.
