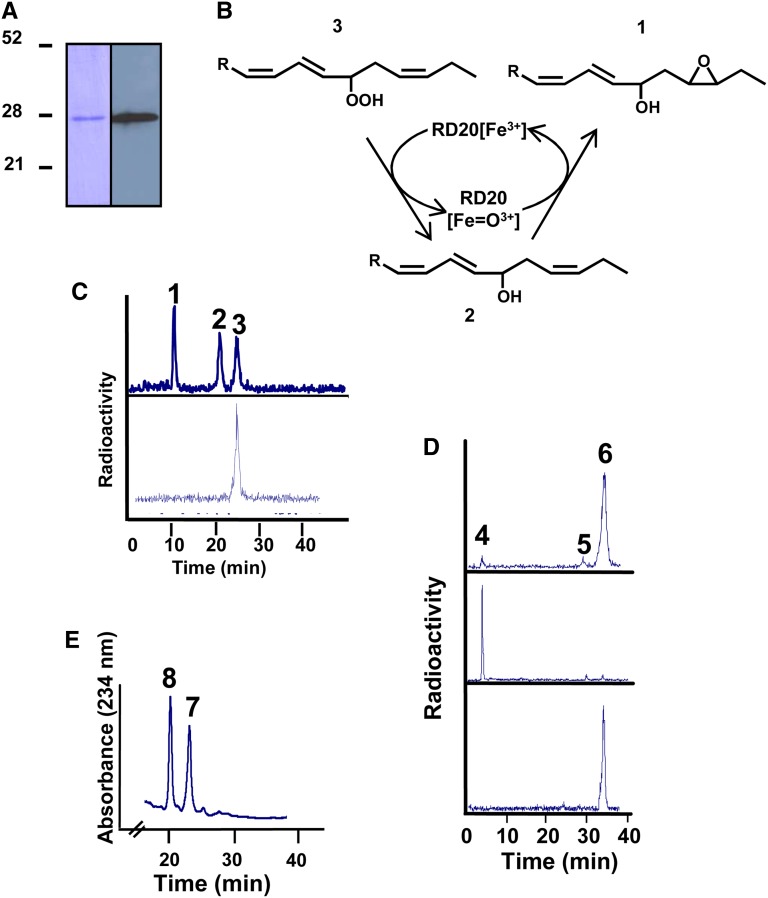
Figure 2.
Characterization of purified recombinant RD20. A, Analysis by SDS-PAGE of a purified fraction of the recombinant protein (left) and western-blot analysis of this fraction using a monoclonal anti-FLAG antibody produced in mouse (right). B, Metabolism scheme showing the products expected from the peroxygenase-catalyzed transformation of FAOOH. C, Radio-HPLC analysis of the metabolization of [1-14C]13-HPOT by purified RD20. Peaks are as follows: 1, 15,16-epoxy-13-hydroxy-9,11-octadecenoic acid; 2, 13-HOT; 3, residual 13-HPOT. The bottom scan shows the absence of 13-HPOT transformation by boiled purified RD20 after 2 h of incubation at 27°C. D, Radio-HPLC analysis of the metabolization of [1-14C]13-HPOD by purified RD20. Top, products of [1-14C]13-HPOD formed by purified RD20 after 2 h of incubation at 27°C. Peaks are as follows: 4, trihydroxy-octadecanoates; 5, 13-hydroxy-9,11-octadecadienoic acid; 6, 13-HPOD. Middle, accumulation of trihydroxy-octadecanoates after overnight incubation of [14C]13-HPOD with purified RD20. Bottom, no epoxy or hydroxy derivatives were formed when [1-14C]13-HPOD was incubated overnight in the presence of a boiled purified RD20. E, Reduction of 9-FAOOH into its corresponding alcohol by purified RD20. Peaks are as follows: 7, 9-HPOT; 8, 9-HOT.