Abstract
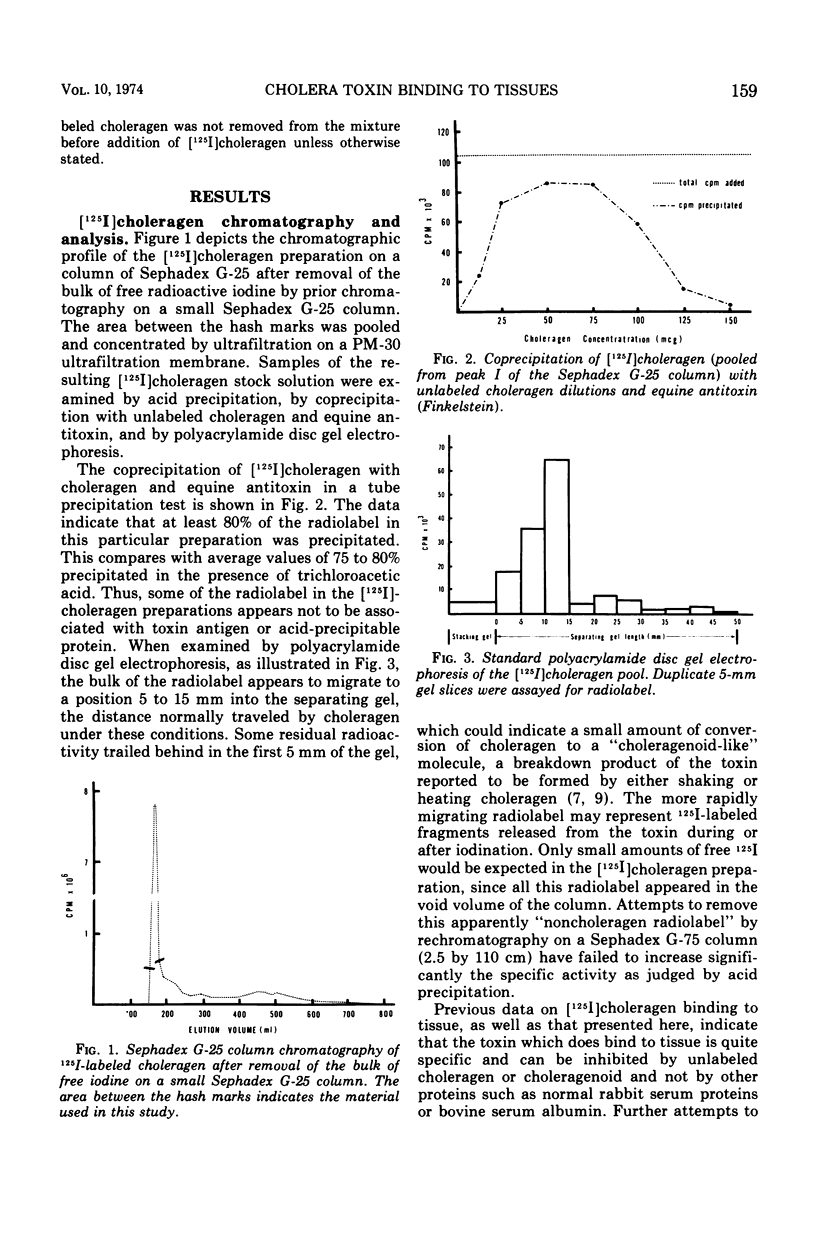
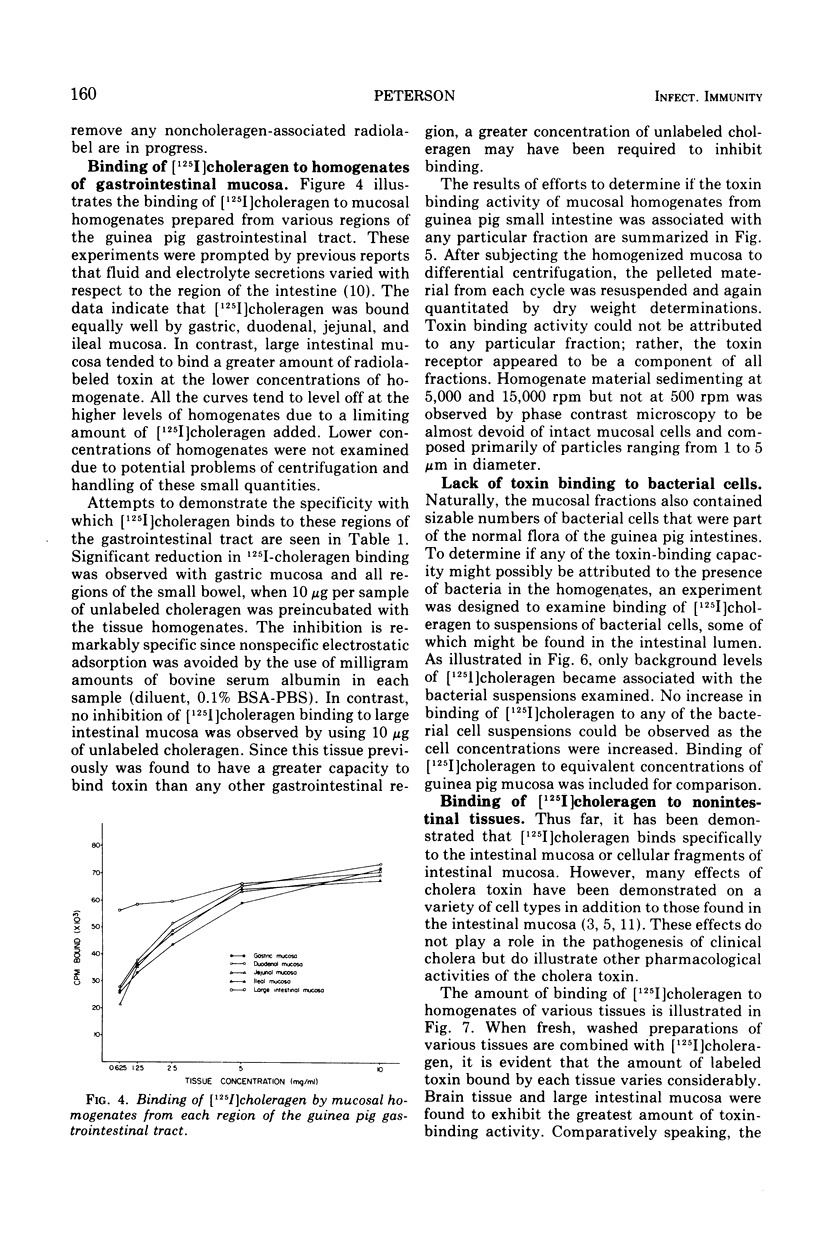
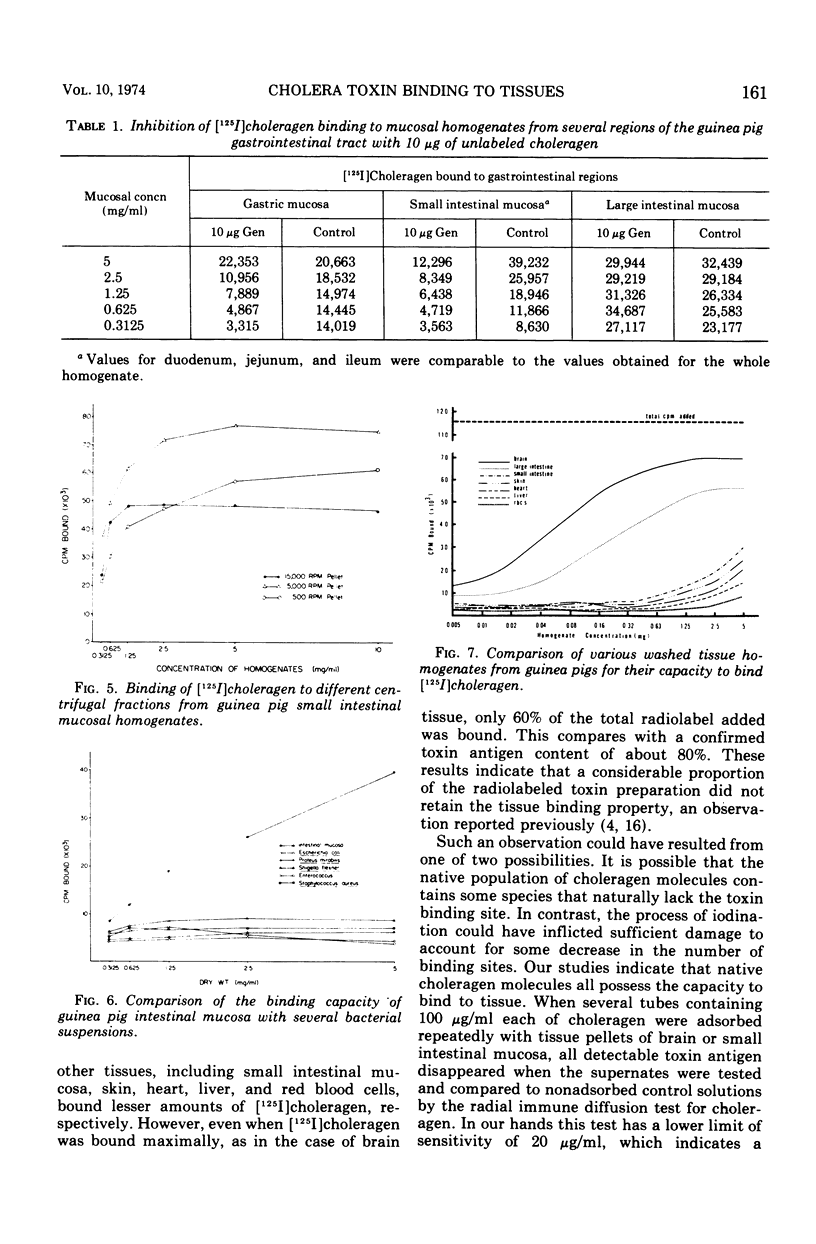
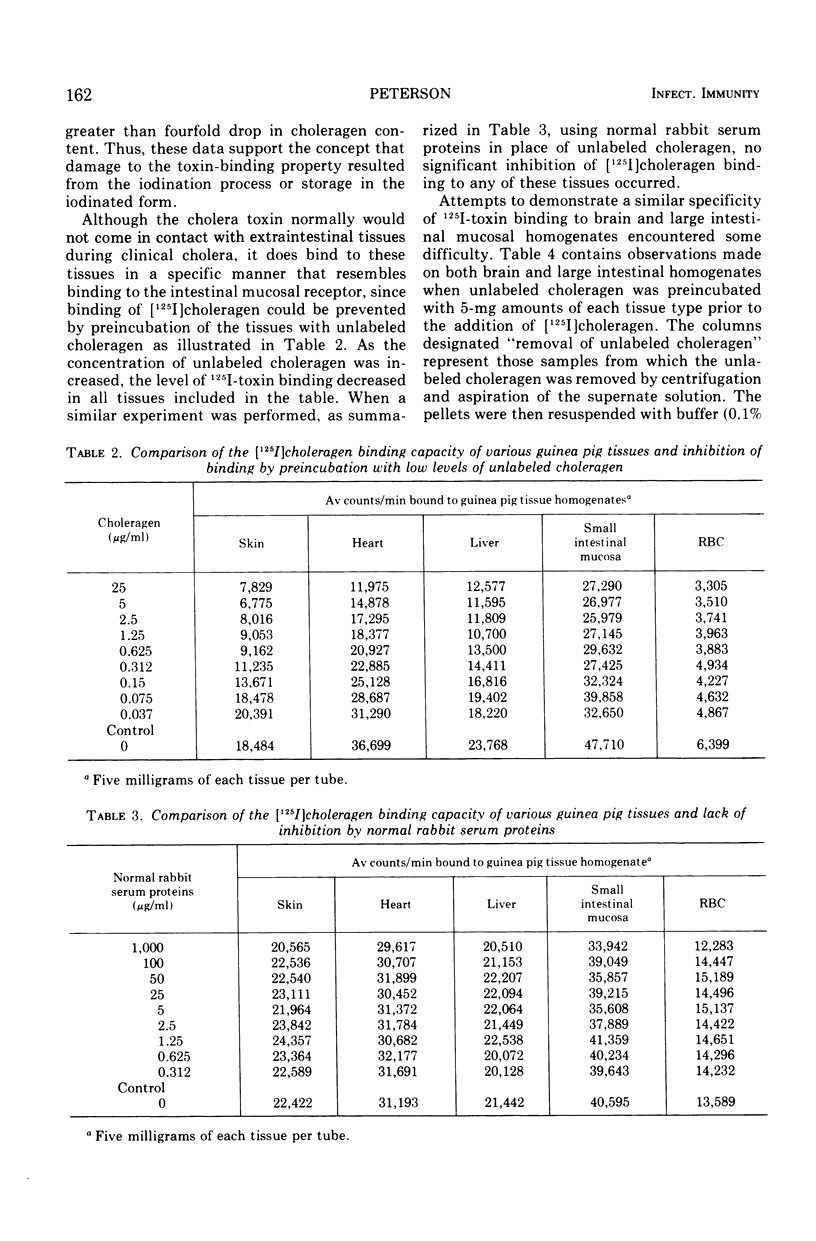
[125I]choleragen was employed to study further the tissue-binding properties of highly purified choleragen. It was observed that [125I]choleragen was bound when combined with mucosal homogenates from all regions of the gastrointestinal tract of adult guinea pigs. Gastric, duodenal, jejunal, and ileal mucosa appeared equally effective in toxin-binding capacity. Preparations of large intestinal mucosa could bind an exceptionally larger amount of toxin. The binding property of small intestinal homogenates could not be attributed to any particular fraction after differential centrifugation; rather, the toxin receptor appeared to be associated with several sizes of particles containing cell membrane components. Although binding to mammalian cells was easily demonstrable, no binding to several types of bacterial cells was observed. The toxin receptor was found to be a “universal component” of many mammalian cell membranes, since specific binding of the toxin to a variety of guinea pig tissues was clearly demonstrated. [125I]choleragen binding to all tissues, with the exception of those prepared from brain and large intestinal mucosa, could be inhibited by preincubation of the tissue homogenates with unlabeled choleragen but not with comparable concentrations of normal rabbit serum proteins. The determination of the specificity of [125I]choleragen binding to brain and large intestinal mucosal homogenates was hampered by the continual release of soluble receptor from the homogenates, both of which contained the highest concentration of cholera toxin receptor. The data support and extend observations that cholera toxin binding to tissue receptor(s) is a very specific reaction, and further indicate that binding may occur with a variety of tissues to different degrees.
Full text
PDF

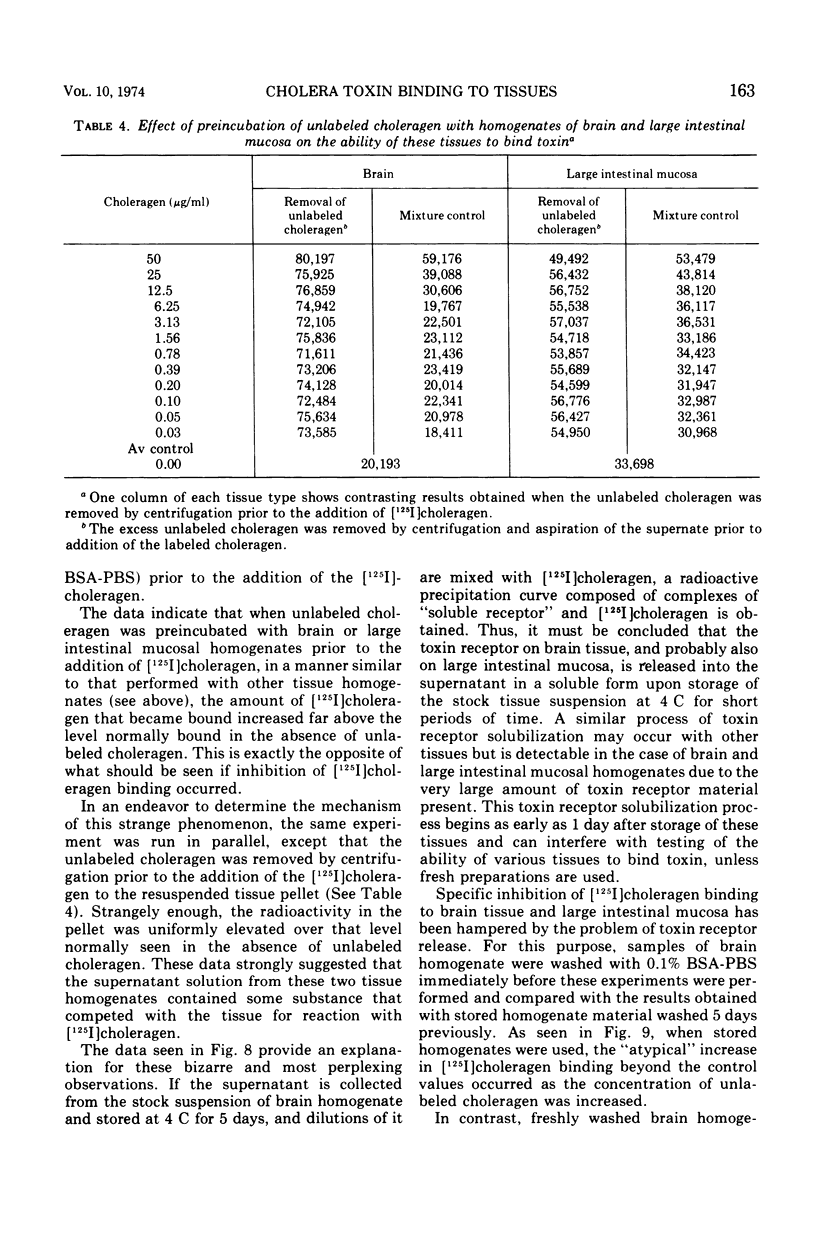
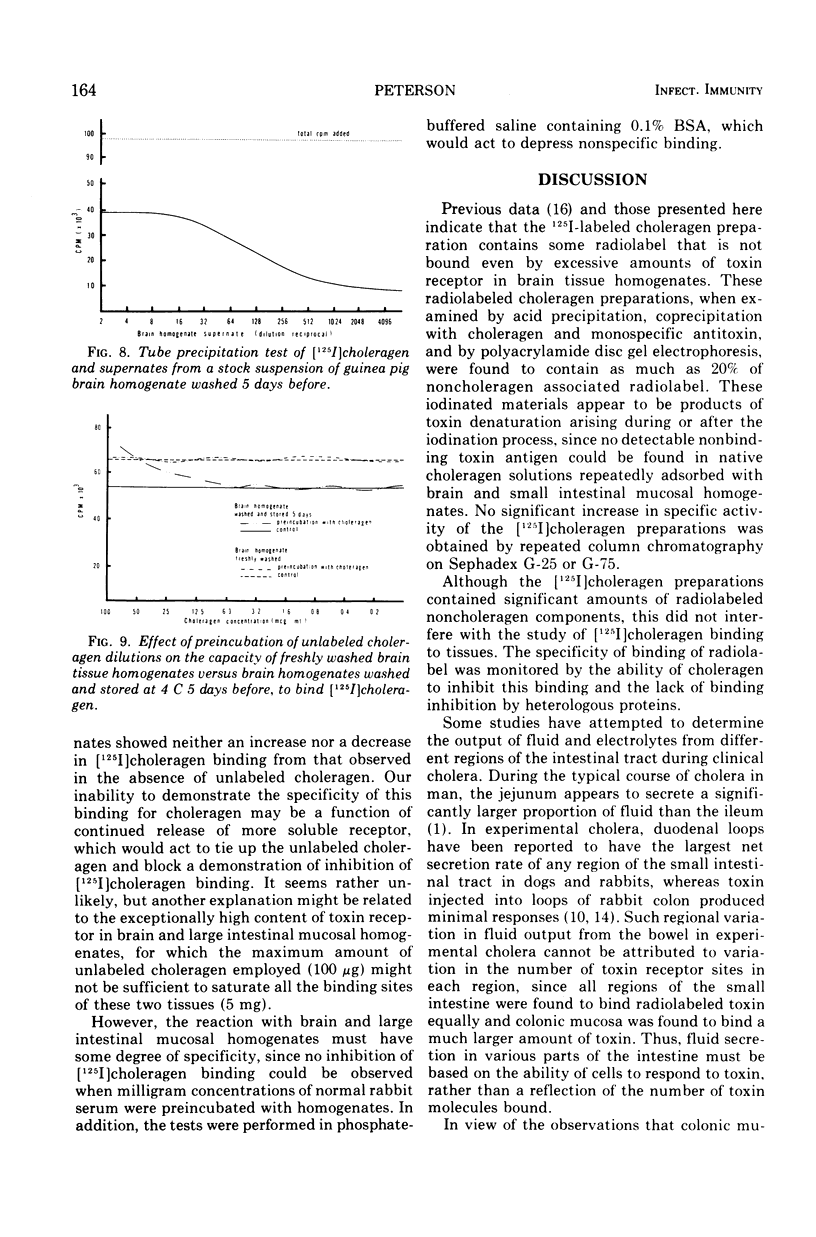


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banwell J. G., Pierce N. F., Mitra R. C., Brigham K. L., Caranasos G. J., Keimowitz R. I., Fedson D. S., Thomas J., Gorbach S. L., Sack R. B. Intestinal fluid and electrolyte transport in human cholera. J Clin Invest. 1970 Jan;49(1):183–195. doi: 10.1172/JCI106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Donta S. T., King M., Sloper K. Induction of steroidogenesis in tissue culture by cholera enterotoxin. Nat New Biol. 1973 Jun 20;243(129):246–247. doi: 10.1038/newbio243246a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A. Monospecific equine antiserum against cholera exo-enterotoxin. Infect Immun. 1970 Dec;2(6):691–697. doi: 10.1128/iai.2.6.691-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Peterson J. W., Lospalluto J. J. Conversion of cholera exo-enterotoxin (choleragen) to natural toxoid (choleragenoid). J Immunol. 1971 Mar;106(3):868–871. [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Leitch G. J., Burrows W. Experimental cholera in the rabbit ligated intestine: ion and water accumulation in the duodenum, ileum and colon. J Infect Dis. 1968 Oct;118(4):349–359. doi: 10.1093/infdis/118.4.349. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., LoSpalluto J. J., Finkelstein R. A. Localization of cholera toxin in vivo. J Infect Dis. 1972 Dec;126(6):617–628. doi: 10.1093/infdis/126.6.617. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Verwey W. F. Radiolabeled toxin for studying binding of cholera toxin and toxoids to intestinal mucosal receptor sites. Proc Soc Exp Biol Med. 1974 Apr;145(4):1187–1191. doi: 10.3181/00379727-145-37978. [DOI] [PubMed] [Google Scholar]
- Van Heyningen W. E., Carpenter C. C., Pierce N. F., Greenough W. B., 3rd Deactivation of cholera toxin by ganglioside. J Infect Dis. 1971 Oct;124(4):415–418. doi: 10.1093/infdis/124.4.415. [DOI] [PubMed] [Google Scholar]


