Abstract
During UV-B-specific induction of bending to the light in Arabidopsis etiolated seedlings, the transcription factor HY5 accumulates on the illuminated side and orchestrates the response.
To date, the main long-standing model explaining phototropic bending is that of Cholodny (1927) and Went (1926), which suggests that the lateral distribution of a growth hormone regulates phototropism and which is supported by a substantial amount of molecular and cell biological evidence (Christie et al., 2011; Ding et al., 2011; Christie and Murphy, 2013). The alternative theory of Blaauw (1919), in which differential growth to unilateral light is thought to occur through unilateral photomorphogenic inhibition of growth, has been pushed into the background, even though supportive data occasionally are published (Yamada et al., 2000). Here, we present further evidence that a mechanism as proposed by Blaauw (1919) can occur in plants as well. We show that UV-B light causes increased accumulation of the transcription factor ELONGATED HYPOCOTYL5 (HY5) at the illuminated side of etiolated Arabidopsis (Arabidopsis thaliana) hypocotyls and that HY5 is necessary for phototropin-independent bending toward UV-B.
Although UV-B radiation has been described as a stressor for plants, low-level exposure leads to typically photomorphogenic responses in the absence of any visible stress (Jansen, 2002). Recently, significant advances in the molecular mechanisms regulating UV-B-specific photomorphogenesis have been achieved thanks to the molecular genetic research opportunities provided by the model plant Arabidopsis (Tilbrook et al., 2013; Jenkins, 2014). The current model for UV-B-specific photomorphogenic signaling states that UV-B is perceived by UV RESISTANCE LOCUS8 (UVR8) homodimer photoreceptor proteins. UVR8 monomerizes and associates with CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1; Favory et al., 2009; Rizzini et al., 2011). By consequence, the transcription factor HY5 is no longer marked for degradation and accumulates (Favory et al., 2009; Huang et al., 2013). In addition, UVR8 and COP1 are known to control UV-B-induced transcription of HY5 (Brown et al., 2005; Oravecz et al., 2006). HY5 activity in general eventually leads to inhibition of hypocotyl elongation (Oyama et al., 1997; Yu et al., 2013).
Apart from general light perception, plants can also sense the direction of incoming light. Many plant species need this response to be able to grow toward the light to obtain favorable conditions for photosynthesis. They have phototropins, which are photoreceptors that stimulate growth toward blue, UV-A, and also UV-B light (Liscum et al., 2014; Vandenbussche et al., 2014). Lack of phototropins confers incapability of growing toward unilateral blue light. However, UV-B through its receptor UVR8 causes bending toward the light in etiolated seedlings, even in the absence of functional phototropins (Vandenbussche et al., 2014).
HY5 MEDIATES DIRECTIONAL BENDING TO UV-B
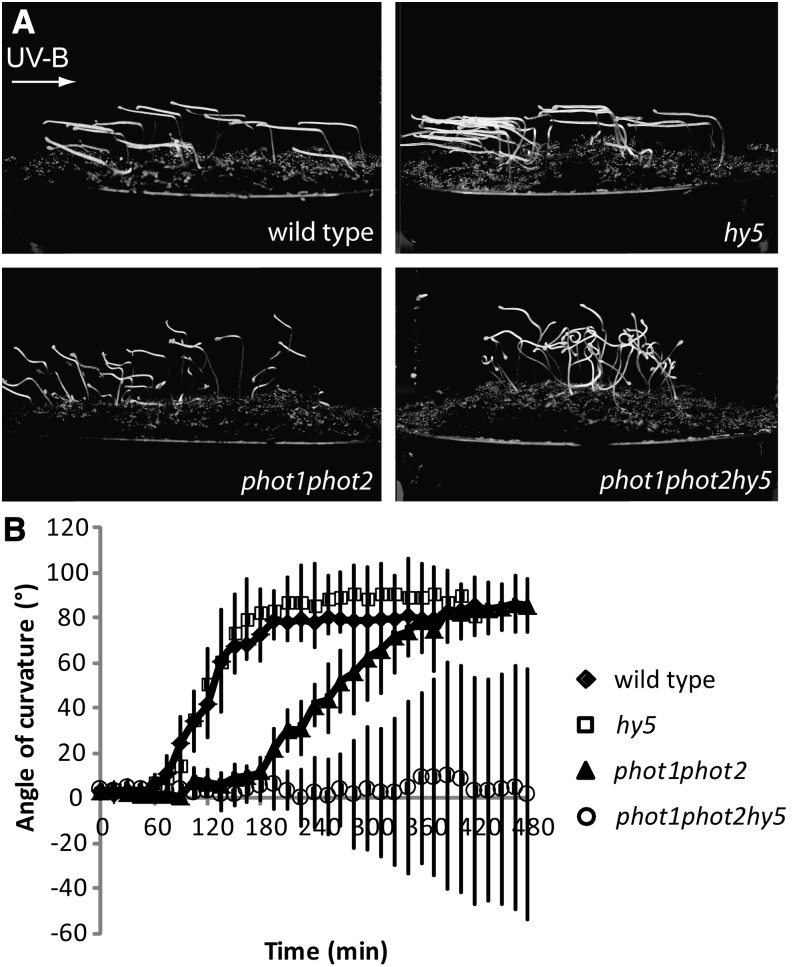
Other than an apparent role for differential auxin signaling, the mechanism of the phototropin-independent UVR8 response is unknown. To unravel the downstream mechanisms of the UVR8 function in these settings, we focused on the known UV-B signaling component HY5 (Ulm et al., 2004; Brown et al., 2005). Etiolated 2-d-old wild-type Columbia, hy5-215 (hereafter referred to as hy5), phototropin1-5 (phot1-5) phot2-1 (hereafter referred to as phot1 phot2), and phot1 phot2 hy5 seedlings were exposed to unilateral monochromatic UV-B light (Vandenbussche et al., 2014). After 24 h of exposure, the wild type, hy5, and phot1 phot2 seedlings showed a clear phototropic response, whereas the phot1 phot2 hy5 triple mutant seedlings did not (Fig. 1A; (Vandenbussche et al., 2014). To study the process in more detail, a kinetic analysis of the seedlings was performed. Pictures of a lateral view of seedlings were taken every 15 min, using a set-up in which the focal plane was parallel to the incoming UV-B light and the bending direction of the wild type. As described previously (Vandenbussche et al., 2014), wild-type plants rapidly oriented toward the incoming light, starting about 1 h after the onset of illumination and completing reorientation after 3 h. hy5 behaved in a very similar way as the wild type, suggesting no major role for HY5 in this rapid bending response (Fig. 1B). Also as described (Vandenbussche et al., 2014), phot1 phot2 still bent toward the light source, yet compared with the wild type, the response was slower, reaching its maximum after about 7 h. By contrast, the phot1 phot2 hy5 triple mutant seedlings did not bend in the direction of the light source. They rather bent in random directions, as indicated by the large standard deviations shown in Figure 1B. These data indicate the importance for HY5 in directional bending toward UV-B light in the absence of a phototropin signal. The results are reminiscent of the response caused by UVR8, which mediates the slower bending response (Vandenbussche et al., 2014). Hence, it is likely that HY5 also in this situation mediates the UVR8 effect. However, another system may cause the randomization of bending, a response associated with phytochromes in other light conditions (Lariguet and Fankhauser, 2004; Kim et al., 2011). In conclusion, bending toward UV-B in etiolated seedlings can be achieved either by a rapid phototropin-dependent response that occurs between 1 and 3 h after onset of exposure or a UVR8-HY5-dependent response that occurs between 3 and 7 h after onset of exposure.
Figure 1.
A, Effect of unilateral UV-B on 2-d-old etiolated seedlings. Seedlings were grown on Jiffy pellets for 2 d in darkness and subsequently exposed to unilateral monochromatic UV-B (0.12 µmol m–2 s–1 of 302 nm). Photographs were taken after 24 h of exposure. B, Kinetic analysis of directional bending in Arabidopsis mutants. Seedlings were grown as in A, with a background of infrared (930-nm) light. Every 15 min, photographs were taken with the focal plane parallel to the direction of UV-B radiation. Quantification of the bending angle was performed on these photographs using ImageJ software (Vandenbussche et al., 2014). Error bars indicate sd (n ≥ 10). wild type, Columbia; hy5, hy5-215; phot1phot2, phot1-5 phot2-1.
UNILATERAL UV-B CAUSES ACCUMULATION OF HY5 AT THE ILLUMINATED SIDE
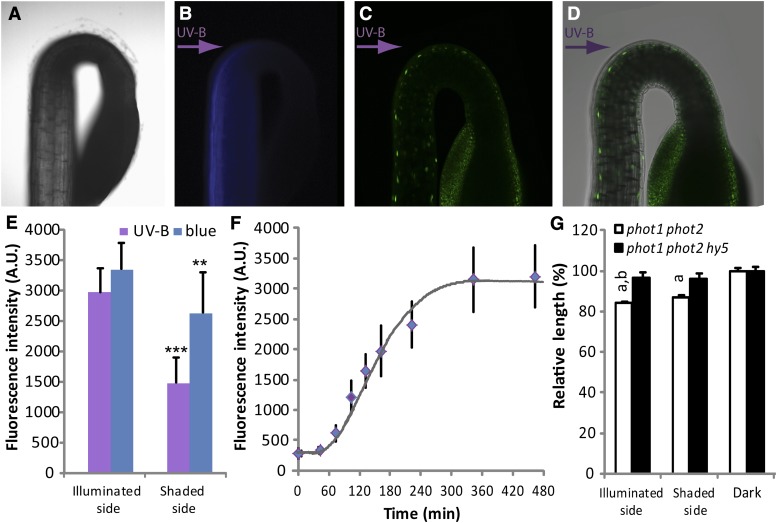
Unilateral UV-B is believed to form a sharp light gradient in seedlings due to reflectance and scattering of incoming light (Vandenbussche et al., 2014). To investigate whether this is the case, etiolated seedlings were stained with the UV-B-excitable optical brightener umbelliferone and subsequently exposed to unilateral UV-B light. Both the wild type (data not shown) and phot1 phot2 mutants were tested (Fig. 2, A and B). There was clearly more fluorescence on the UV-B illuminated side than on the shaded side (Fig. 2B), confirming the existence of a gradient of UV-B light in the seedling. However, to be meaningful for a plant, a light gradient somehow needs to be functional. The HY5 gene is an excellent marker for UV-B signaling, as it is both transcriptionally induced and stabilized at protein level in the presence of UV-B light (Ulm et al., 2004; Brown et al., 2005; Favory et al., 2009; Huang et al., 2013). In addition, HY5 is considered an integral part of UV-B photomorphogenic signaling (Tilbrook et al., 2013). Therefore, to analyze the phototropin-independent, HY5-dependent phototropic response in more detail, accumulation of the HY5 protein was examined. The effect of unilateral UV-B exposure on HY5-Yellow Fluorescent Protein (YFP) under the natural HY5 promoter (Oravecz et al., 2006) was analyzed under confocal microscope in phot1 phot2 background. The illuminated side clearly accumulated more HY5-YFP protein (Fig. 2, C and D). This response was much stronger in UV-B than in blue light. The blue light-induced accumulation of HY5 in this case is likely to depend on blue light perceiving photoreceptors such as cryptochromes or phytochromes (Osterlund et al., 2000; Wang et al., 2001) and appears less differential. Lack of a blue light phototropic response in phot1phot2 mutants (Sakai et al., 2001) also suggests that the smaller differentiality in HY5 accumulation formed by blue light is not sufficient to induce phototropic bending. In a more detailed analysis, the HY5-YFP accumulation at the illuminated side in UV-B was followed in time by monitoring nuclear fluorescence (Pacín et al., 2014). Quantification showed that no significant difference with the dark control was observed after 1 h of illumination (Fig. 2F). At this point, the phototropin-dependent response (Fig. 1; Vandenbussche et al., 2014) has already started. Thus, the timing of accumulation of HY5 in UV-B appeared slightly later than the start of output of phototropin action, yet preceded the bending response in phot1 phot2 mutants (compare Fig. 2F with Fig. 1). The rate of accumulating of HY5 is comparable to that in white light (Pacín et al., 2014). Thus, the accumulation of HY5 seems sufficiently early for this transcription factor to generate eventual phenotypic output such as the directional bending. Together, the data support a model in which UV-B causes unilateral accumulation of HY5, which then, in the end, leads to bending toward the light.
Figure 2.
UV-B-induced accumulation of HY5. A, Bright-field image of the top of a 2-d-old etiolated phot1 phot2 seedling. B, Blue fluorescence in the seedling (as in A) illuminated with monochromatic UV-B (0.12 µmol m–2 s–1 of 306 nm from the left). The seedling was stained in 100 µm umbelliferone in water, rinsed three times, mounted on a cover slip, and imaged using a Zeiss Axiovert microscope, while being exposed to unilateral UV-B. C, Confocal laser-scanning microscopic (Nikon EZC1) image from a phot1 phot2 seedling containing the pHY5::HY5-YFP construct. The seedling was grown for 2 d in darkness and then exposed to unilateral monochromatic UV-B (0.12 µmol m–2 s–1 of 302 nm, from the left) for 270 min. Nuclear HY5-YFP accumulation is visible as bright green spots. D, Confocal laser-scanning microscopic image, as in C, with differential interference contrast image overlay. E, Quantification of fluorescence in populations of seedlings grown as in C. Unilateral light was either 0.12 µmol m–2 s–1 of 302 nm (UV-B) or 450 nm (blue). Imaging was completed within 10 min after harvesting. Fluorescence intensity in nuclei of epidermal cells in the growth zone was measured at the illuminated and the shaded side using Nikon Instruments elements Advanced Research analysis software. Error bars indicate sd on fluorescence from different seedlings (n > 10). Statistical significance versus illuminated side: **P < 0.01, ***P < 0.001. F, Kinetic analysis of HY5-YFP accumulation upon unilateral UV-B illumination (0.12 µmol m–2 s–1 of 302 nm) in 2-d-old etiolated phot1 phot2 pHY5::HY5-YFP seedlings during the first 8 h after exposure. Fluorescence measurement was done as in D, using fixed acquisition settings of the 480-min sample, which was adjusted to yield values just under saturation. Error bars indicate sd on fluorescence from different seedlings (n > 5). G, Seedlings were grown for 2 d in darkness and then exposed to 18 h of unilateral monochromatic UV-B (0.12 µmol m–2 s–1 of 302 nm) or kept in darkness. Photographs were taken, and hypocotyl length was measured using ImageJ software. Data were normalized to the length of dark-grown seedlings. a, significant (P < 0.05) difference with dark control; b, significant (P < 0.05) difference with the length of the shaded side. Error bars represent se of the mean (n ≥ 25).
Considering the importance of HY5 for elongation of the hypocotyl, we tested whether HY5 in UV-B causes a stronger inhibition of growth at the illuminated side compared with the shaded side. After UV-B exposure, the length of phot1 phot2 hypocotyls was shorter at the illuminated side, while this was not the case in phot1 phot2 hy5 triple mutants (Fig. 2G). It was shown before that auxin is involved in the UVR8-dependent directional bending. Thus, it is tempting to speculate that HY5 mediates local auxin signal reduction at the illuminated side, either by inhibiting signaling or stimulating auxin efflux, or both. In case efflux is involved, auxin transport toward the shaded side may exist and both the Cholodny-Went and Blaauw theories apply, unifying both hypotheses. Notably, HY5 has been suggested to negatively regulate auxin signaling (Sibout et al., 2006), and auxin has a role in UVR8-dependent bending (Vandenbussche et al., 2014). Furthermore, regulation of tropic responses has been linked to both auxin transport and signaling (Kami et al., 2014).
The biological role of the UV-B-specific response is still under investigation, but it could be important in situations in which the phototropin response is nearly absent or near saturation. With the data presented here, the UVR8- and HY5-regulated photomorphogenic pathway fits in a scenario where differential growth is regulated according to the Blaauw theory, indicating that both the Cholodny-Went and the Blaauw models for directional growth can apply in plants.
Acknowledgments
We thank Roman Ulm for providing the phot1 phot2 hy5 triple mutant seeds and HY5::HY5-YFP and critical reading of the manuscript.
Footnotes
This work was supported by the Research Foundation Flanders (grant no. 1514912 to F.V.) and research projects from the Research Foundation Flanders (nos. G.0298.09 and G.0656.13 to D.V.D.S.) and Ghent University (to D.V.D.S.).
References
- Blaauw AH. (1919) Licht und Wachstum III. Mededelingen Landbouwhogeschool Wageningen 15: 89–204 [Google Scholar]
- Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI. (2005) A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci USA 102: 18225–18230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholodny N. (1927) Wuchshormone und Tropismem bei den Pflanzen. Biol Zent Bl 47: 604–626 [Google Scholar]
- Christie JM, Murphy AS. (2013) Shoot phototropism in higher plants: new light through old concepts. Am J Bot 100: 35–46 [DOI] [PubMed] [Google Scholar]
- Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, et al. (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski Ł, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, et al. (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, et al. (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 28: 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ouyang X, Yang P, Lau OS, Chen L, Wei N, Deng XW. (2013) Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc Natl Acad Sci USA 110: 16669–16674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK. (2002) Ultraviolet-B radiation effects on plants: induction of morphogenic responses. Physiol Plant 116: 423–429 [Google Scholar]
- Jenkins GI. (2014) The UV-B photoreceptor UVR8: from structure to physiology. Plant Cell 26: 21–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami C, Allenbach L, Zourelidou M, Ljung K, Schütz F, Isono E, Watahiki MK, Yamamoto KT, Schwechheimer C, Fankhauser C. (2014) Reduced phototropism in pks mutants may be due to altered auxin-regulated gene expression or reduced lateral auxin transport. Plant J 77: 393–403 [DOI] [PubMed] [Google Scholar]
- Kim K, Shin J, Lee SH, Kweon HS, Maloof JN, Choi G. (2011) Phytochromes inhibit hypocotyl negative gravitropism by regulating the development of endodermal amyloplasts through phytochrome-interacting factors. Proc Natl Acad Sci USA 108: 1729–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P, Fankhauser C. (2004) Hypocotyl growth orientation in blue light is determined by phytochrome A inhibition of gravitropism and phototropin promotion of phototropism. Plant J 40: 826–834 [DOI] [PubMed] [Google Scholar]
- Liscum E, Askinosie SK, Leuchtman DL, Morrow J, Willenburg KT, Coats DR. (2014) Phototropism: growing towards an understanding of plant movement. Plant Cell 26: 38–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oravecz A, Baumann A, Máté Z, Brzezinska A, Molinier J, Oakeley EJ, Adám E, Schäfer E, Nagy F, Ulm R. (2006) CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 18: 1975–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MT, Hardtke CS, Wei N, Deng XW. (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K. (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacín M, Legris M, Casal JJ. (2014) Rapid decline in nuclear costitutive photomorphogenesis1 abundance anticipates the stabilization of its target elongated hypocotyl5 in the light. Plant Physiol 164: 1134–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, et al. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106 [DOI] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R, Sukumar P, Hettiarachchi C, Holm M, Muday GK, Hardtke CS. (2006) Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet 2: e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbrook K, Arongaus AB, Binkert M, Heijde M, Yin R, Ulm R. (2013) The UVR8 UV-B photoreceptor: perception, signaling, and response. Arabidopsis Book 11: e0164, /10.1199/tab.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R, Baumann A, Oravecz A, Máté Z, Adám E, Oakeley EJ, Schäfer E, Nagy F. (2004) Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci USA 101: 1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Tilbrook K, Fierro AC, Marchal K, Poelman D, Van Der Straeten D, Ulm R. (2014) Photoreceptor-mediated bending towards UV-B in Arabidopsis. Mol Plant 7: 1041–1052 [DOI] [PubMed] [Google Scholar]
- Wang H, Ma LG, Li JM, Zhao HY, Deng XW. (2001) Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294: 154–158 [DOI] [PubMed] [Google Scholar]
- Went FW. (1926) On growth accelerating substances in the coleoptile of Avena sativa. Proc Kon Ned Akad Wet 30: 10–19 [Google Scholar]
- Yamada K, Nakano H, Yokotani-Tomita K, Bruinsma J, Yamamura S, Hasegawa K. (2000) Repetition of the classical Boysen-Jensen and Nielsen’s experiment on phototropism of oat coleoptiles. J Plant Physiol 156: 323–329 [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang J, Zhang Z, Quan R, Zhang H, Deng XW, Ma L, Huang R. (2013) Ethylene promotes hypocotyl growth and HY5 degradation by enhancing the movement of COP1 to the nucleus in the light. PLoS Genet 9: e1004025. [DOI] [PMC free article] [PubMed] [Google Scholar]