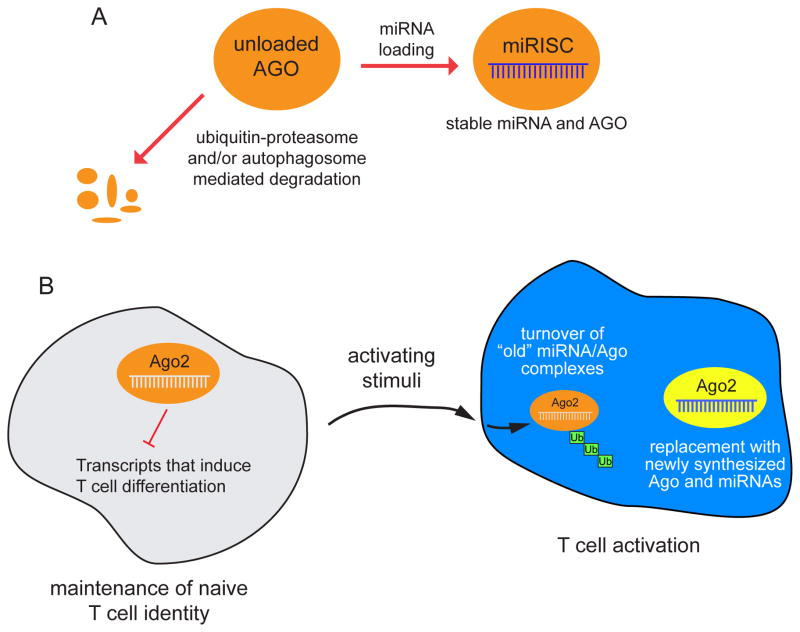
Figure 2.
Models for how ubiquitination can affect Ago proteins. (Top) Unloaded Ago proteins in many cellular contexts are susceptible to degradation by ubiquitin-proteasome and/or autophagosome-dependent pathways. These mechanisms adjust the level of Ago proteins in concert with miRNA biogenesis. (Bottom) During T cell activation, the ubiquitination-dependent turnover of Ago2 is associated with overall downregulation of miRNA accumulation. This may facilitate the re-setting of a different miRNA repertoire in activated T cells, in which the “old” miRNA/Ago pool is actively degraded and replaced with newly transcribed miRNAs and newly synthesized Ago proteins.