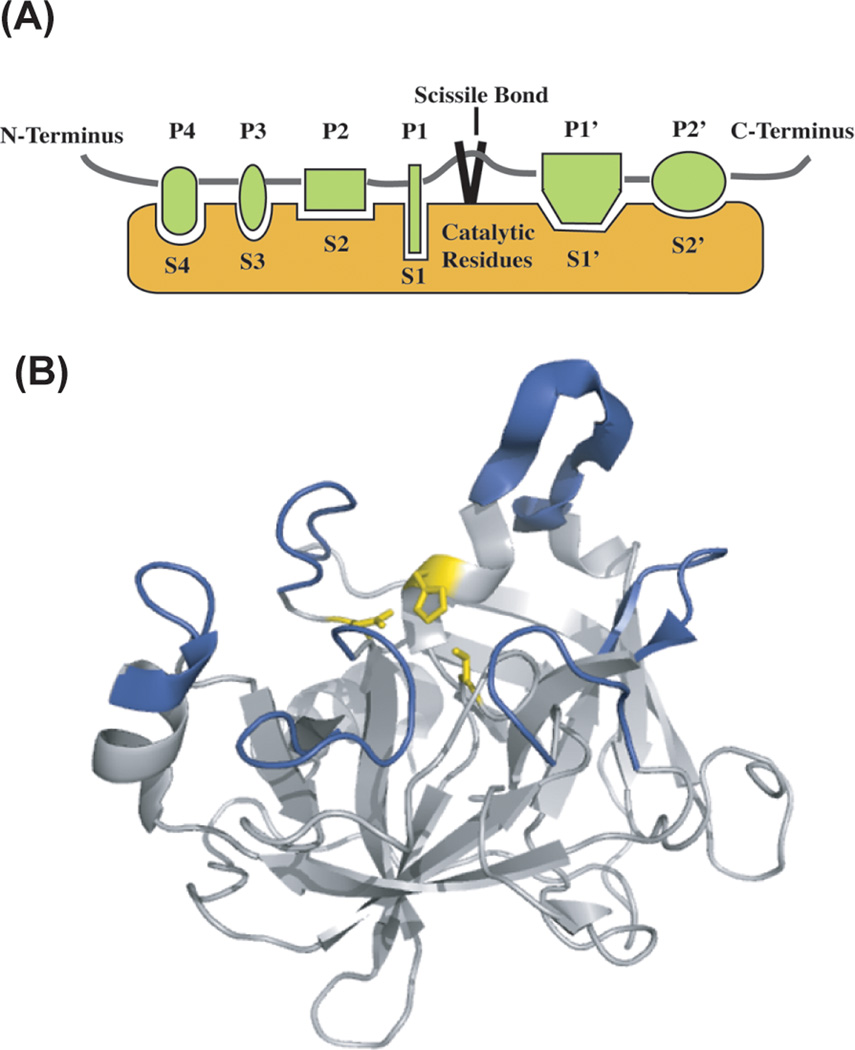
Figure 1.
(A) Diagram of a protease active site. A protease cleaves a peptide at the scissile bond, and has a number of specificity subsites, which determine protease specificity. Substrates bind to a protease with their non-prime residues on the N-terminal side of the scissile bond and their prime-side residues C-terminal to the scissile bond. The catalytic residues determine the class of protease. Serine, cysteine, and threonine proteases hydrolyze a peptide bond via a covalent acyl-enzyme intermediate, and aspartic, glutamic and metalloproteases activate a water molecule to hydrolyze the peptide bond in a non-covalent manner. (B) A serine protease (matriptase/MT-SP1, 1EAX.pdb) with the catalytic triad in yellow and the surface loops that surround the active site colored in blue. While the catalytic architecture of proteases is remarkably conserved, the surface loops are areas of high sequential and structural diversity.