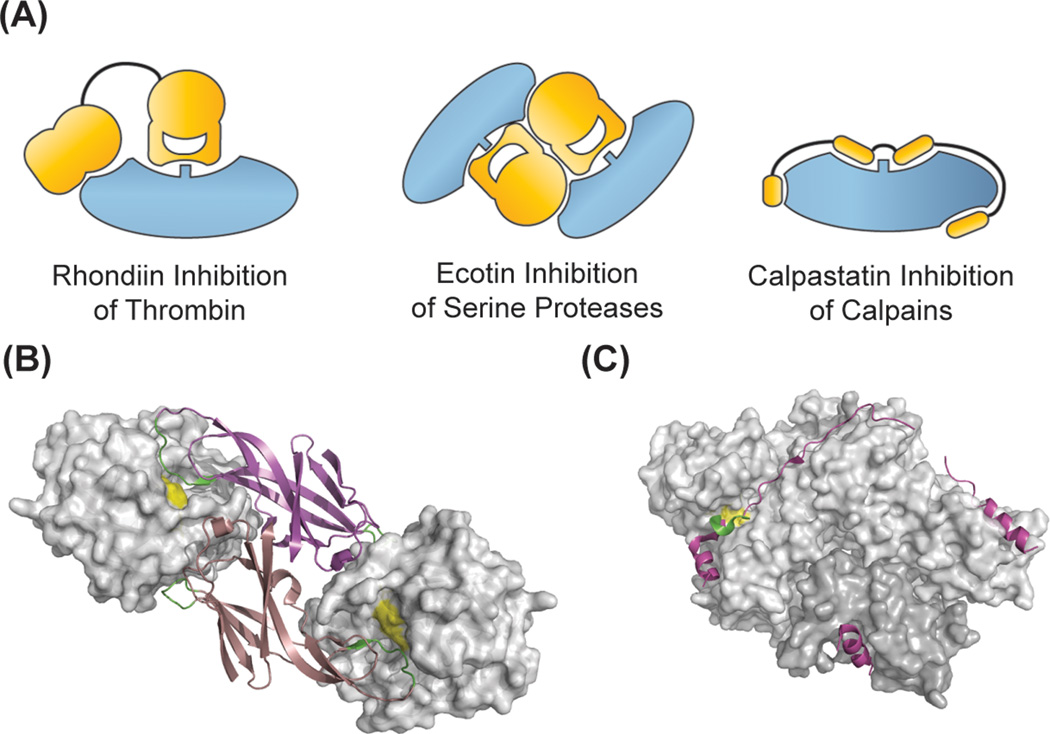
Figure 3.
Inhibitors that take advantage of exosite binding. Most exosite inhibitors are competitive inhibitors that prevent substrate binding at the active site. In the case of (B) ecotin (bound to trypsin, 1EZU.pdb), the exosites provide binding energy and allow for broad specificity, while (C) calpastatin gains binding energy and specificity by forming critical interactions across the calpain protease surface (3BOW.pdb).