Abstract
J wave syndrome has emerged as a significant cause of Idiopathic ventricular fibrillation (IVF) responsible for sudden cardiac death. A large body of data is now available on genesis, genetics and ionic mechanisms of J wave syndromes. Two of these viz., Early repolarization syndrome (ER) and Brugada syndrome (BrS) are fairly well characterized enabling correct diagnosis in most patients. The first part of repolarization of ventricular myocardium is governed by Ito current i.e., rapid outward potassium current. The proposed mechanism of ventricular fibrillation (VF) and ventricular tachycardia (VT) storms is the faster Ito current in the epicardium than in the endocardium results in electrical gradient that forms the substrate for phase 2 reentry. Prevention of Ito current with quinidine supports this mechanism. Majority of ER patterns in young patients are benign. The key issue is to identify those at increased risk of sudden cardiac death. Association of both ER syndrome and Brugada syndrome with other disease states like coronary artery disease has also been reported. Individuals resuscitated from VF definitely need an implantable cardiac defibrillator (ICD) but in others there is no consensus regarding therapy. Role of electrophysiology study to provoke ventricular tachycardia or fibrillation is not yet well defined. Radiofrequency ablation of epicardial substrate in right ventricle in Brugada syndrome is also under critical evaluation. In this review we shall discuss historical features, epidemiology, electrocardiographic features, ionic pathogenesis, clinical features and current status of proposed treatment of ER and BrS.
Keywords: Fibrillation, Tachycardia, Early repolarization syndrome (ER), J wave syndrome
1. Introduction
Syndrome word comes from Latin/Greek and means syndrome, literally: a running together, (from syn- + dramein to run). In medicine it is defined as any combination of signs and symptoms that are indicative of a particular disease or disorder with multiple etiologies. J wave syndrome is a recently recognized entity that is being defined, and perhaps encompasses more than one condition. The letter ‘J’ means junction and actually here it defines the junction point of QRS with ST segment.1 Electrical activity recorded by electrocardiogram (ECG) is a recording of action potentials (AP) resulting from movement of ions across myocardial membrane causing depolarization (contraction of myocardial sarcomere), followed by return of ions to basal state causing repolarization (relaxation). This action potential has five components labeled as phases 0–4, 0 being depolarization and 1–4 repolarizations. Junction point of end of depolarization and onset of repolarization is the J-point on ECG and on the action potential curve. Ion movement is through pores/channels made up of proteins that are regulated by genes. Disorders caused by altered channels/ion movements known as channelopathies are possibly monogenetic syndromes.2,3
Two important J wave syndromes have been described viz., Early repolarization syndrome (ER) and Brugada syndrome (BS). We shall discuss about pathophysiology, clinical symptom constellation, diagnostic methods and treatment of these conditions.1
2. Genesis of abnormal action potential
Inward movement of Na across the cell membrane through a specific channel produces inward current and causes depolarization. Outward movement of K causes repolarization. There are 8 types of Potassium (K) currents. The plateau phase of action potential AP depends on balance between inward (depolarizing) and outward (repolarizing) currents. Ventricular myocardium has actually three layers of muscles viz., epicardial, M cells and endocardial layer. To some extent the M cells resemble Purkinje cells. In normal state there is homogenous genesis of action potential across the three layers (Fig. 1). Abnormality of any layer would lead to aberration in action potential and development of ionic gradient between the layers with consequent development of slow conduction, forming a substrate for reentry in phase 2. The first phase of repolarization with a dome like small hump is due to K current called Ito (transient outward current) (Fig. 2). Uneven distribution with resultant gradient of Ito between epicardium and endocardium results in J-point elevation. Hypothermia and hypocalcaemia enhance J wave development.2
Fig. 1.
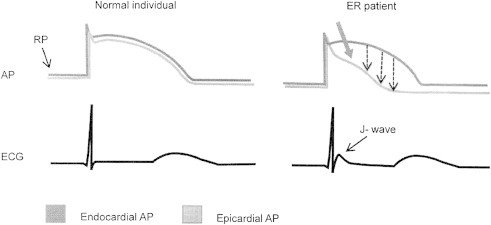
Possible mechanism of J wave genesis. Action potential in normal and J wave syndrome.
Fig. 2.
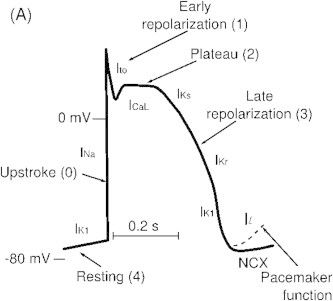
Ito responsible for ER.
3. Early repolarization syndrome
ER is characterized by prominent J-point on ECG with notching/slurring of distal part of R wave which more or less appears as pseudo delta wave. J notches are known for long time and are actually present in 2–10% of general population. J wave attracted significant clinical attention following publication of Haissaguerre et al,4 where they showed relationship of Idiopathic Ventricular Fibrillation with ER. In this case controlled study, young patients who were successfully resuscitated from idiopathic ventricular fibrillation were carefully reviewed, their baseline ECG revealed prominent J waves in inferolateral leads which could not be explained by the trauma of sudden cardiac arrest, resuscitation efforts or drugs used for resuscitation. Considerable interest was generated following this landmark paper, and benign versus malignant varieties of ER were described. The former is an asymptomatic ECG finding in general population, while the latter presents with history of ventricular fibrillation.
4. History of J wave recognition
The pattern of ER and J deflection presenting as slurring or notching of the terminal part of QRS complex was first described in 1936 by Shipley and Hallaran and was considered a normal ECG variant.5 In 1938, Tomaszewski presented the case of an accidentally frozen man whose ECG depicted a very slowly inscribed deflection between the QRS complex and the ST segment, representing a J wave.6 In 1953, Osborn described a “current of injury” later named “the Osborn wave” in acidotic and hypothermic dogs at rectal temperatures less than 25 °C.7
In 1961,Wasserburger et al further defined early repolarization as a 1–4 mm takeoff of the ST segment from the isoelectric line accompanied by downward concavity of the ST segment and symmetrically limbed T wave often of large amplitude in the mid to left precordial leads.8
In 1984, Otto et al described three cases of Ventricular fibrillation (VF) with structurally normal hearts that showed ER to be no more benign. They presented three cases of VF that occurred during sleep in young male Southeast Asian refugees who had structurally normal heart and the only prominent ECG abnormality in these patients was a prominent J wave accompanied by ST segment elevation.9
In 1999, Gussak and Antzelevitch suggested that early repolarization may be malignant in some cases, based on observations that an early repolarization pattern in arterial perfused wedge preparations can easily convert to one in which phase 2 reentry gives rise to polymorphic ventricular tachycardia (VT)/VF.10
In 2000, evidence supporting above hypothesis was provided by Kalla et al and Takagi et al; they reported VF in patients with prominent J wave and ST segment elevation in inferior leads without structural heart diseases and postulated that idiopathic VF with an early repolarization pattern in inferior leads may represent a variant of the Brugada syndrome.11,12
In 2008, Haissaguerre et al4 and Nam et al13 demonstrated a definitive association between J waves and the pathogenesis of many different forms of idiopathic VF.
5. Types of early repolarization (ER)
Antzelevitch et al described early repolarization syndrome into three subtypes15:
Type 1: Early repolarization pattern predominantly in the lateral precordial leads. This form is very prevalent among healthy male athletes and is rarely seen in VF survivors.
Type 2: Early repolarization pattern predominantly in the inferior sor inferolateral leads. Numerous cases of otherwise idiopathic VF have this ECG pattern, this is also prevalent in healthy young males.
Type 3: Early repolarization pattern globally in the inferior, lateral, and right precordial leads and is associated with the highest level of risk for development of malignant arrhythmia. It is often associated with VT/VF storms.
Tikkanen et al.15 proposed another classification (Fig 3). ER is J-point and ST segment elevation >1 mm in 2 or more contiguous leads. Two types of J-point elevation are described:
-
1.
J-point with rapidly ascending ST segment, considered a Benign form.
-
2.
J-point with horizontal or descending ST segment, considered a Malignant form.
Fig. 3.
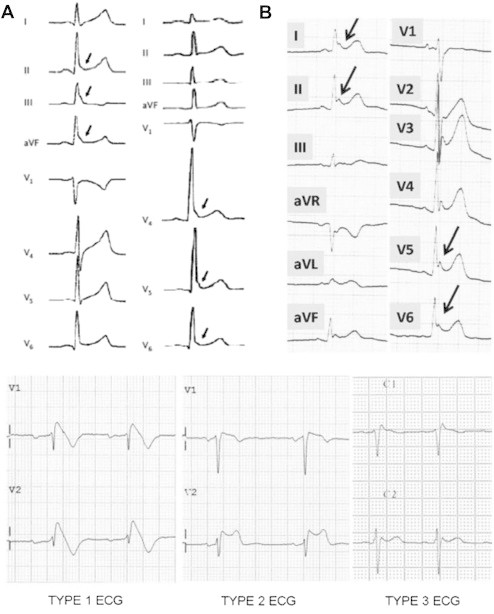
A. Benign type with rapidly ascending ST and B. Malignant type with horizontal variety of ER.
ER in inferolateral lead is more associated with ventricular fibrillation.16 ER ECG pattern (>1 mm) in the inferior/lateral leads occurs in 1–13% of the general population and in 15–70% of Idiopathic VF cases. In the pediatric age group it is even more prevalent. Male sex is strongly associated with ER ECG pattern, since over 70% of subjects with ER are males, its prevalence declines in males from early adulthood until middle-age which suggests a hormonal influence on the presence of ER. The ER pattern is more common in young physically active individuals, athletes, and African-Americans. There is an increased prevalence of ER reported in Southeast Asians. The ER pattern is associated with high vagal tone, as well as hypothermia and hypocalcaemia.17
6. Clinical diagnosis
Following clinical patterns are now known14,15:
-
1.
Asymptomatic and incidentally detected ER is very common in young athletes. The prevalence and magnitude of ER increase as their training intensifies.
-
2.
Malignant variety with Idiopathic VF and Sudden cardiac death (SCD).
-
3.
ER with Coronary artery disease (CAD) with increased risk of having ischemic VF. ER pattern recorded during ischemic event is strongest predictor of VF occurrence.
-
4.
ER has been linked to high cardiac death and arrhythmic death rates in vasospastic angina.
-
5.
Idiopathic VF is reported with horizontal or down-sloping ST following J-point elevation (Fig. 3).
7. Genetic basis and variants
The genetic basis for early repolarization is not well defined. Genetic contributions to ER are suggested by anecdotal observations of a common familial history of SCD in subjects with ER and idiopathic VF. Familial ER has been reported to have an autosomal dominant inheritance pattern with incomplete penetrance. Two independent population-based studies also have suggested some degree of inheritance of the ER patterns in the general population, but the familial inheritance of malignant ER patterns has not been clearly demonstrated. A candidate gene approach in idiopathic VF patients with ER has identified a mutation in KCNJ8, which encodes a pore-forming subunit of the ATP-sensitive potassium channel.
Mutations in the L-type calcium channel genes, including CACNA1C, CACNB2B, and CACNA2D1 as well as loss-of-function mutations in SCN5A have also been associated with idiopathic VF with ER. Given the high prevalence of ER in the general population, ER likely has a polygenic basis that also is influenced by non-genetic factors.18–20
8. Diagnosis
There are no validated techniques to provoke the ER pattern, although 12-lead Holter monitoring to detect evidence of the ER pattern during bradycardia is warranted. In survivors of VF and in patients with polymorphic VT, clinical evaluation to rule out structural heart disease including echo-cardiogram, coronary angiography, magnetic resonance imaging (MRI), and in selected cases, endomyocardial biopsies should be performed to exclude other causes of VF.
9. Risk stratification
The magnitude of the J-point elevation may have prognostic significance (Figs. 4 and 5). Either slurred or notched J-point elevation ≥0.2 mV is relatively rare in the general population, but appears to be associated with an increased risk. Furthermore, J-point elevation in idiopathic VF patients is of greater amplitude and ECG lead distribution compared to those with an established cause of cardiac arrest. The available data also suggest that transient changes in the presence and amplitude of J-point elevation portend a higher risk for VF. A horizontal or descending ST segment following J-point elevation is associated with a worse outcome in the general population. This observation has been very helpful in distinguishing idiopathic VF.14–16
Fig. 4.
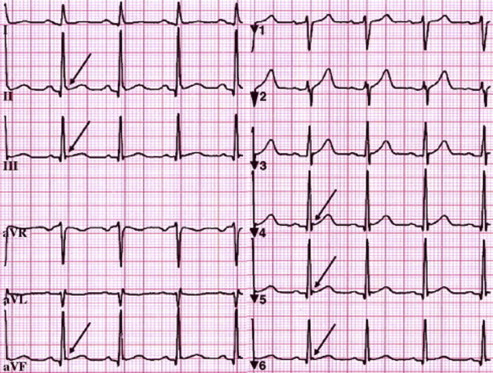
ER with J-point elevation in Inferior and lateral leads.
Fig. 5.
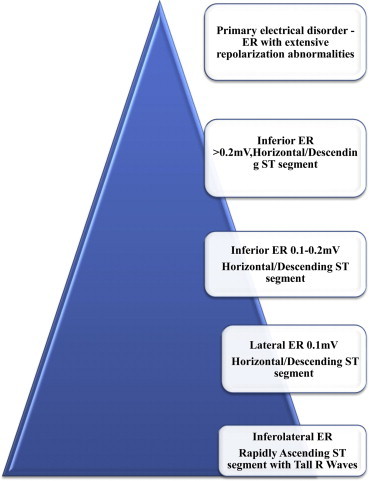
Inferolateral early repolarization patterns and magnitude of risk of sudden cardiac death. Estimated prevalence in the general population is manifested by width of the pyramid. Highest risk is on the top of the pyramid, and lowest on the bottom (Adapted from Junttila MJ et al15).
10. Management
The clinical implications of the observation of an ER pattern in the ECG of an asymptomatic subject are not clear at this stage. The presence of ER is associated with 3 times the risk of developing VF, but the overall risk is still negligible considering the rarity of VF in the general population. Because the presence of ER may increase the vulnerability to sudden death during an acute ischemic event, a plausible implication coming from the population studies is that middle-aged subjects with the ER pattern in the ECG, especially those with a high amplitude of J-point elevation and horizontal (≥2 mm) or down-sloping ST segment, should target a reduction in their long-term risk for acute coronary events in accordance with current practice guidelines. Electrical storm is relatively common after ICD implantation in patients with the ER syndrome. Case series evidence supports the acute use of isoproterenol for suppression of recurrent VF, and quinidine for long-term suppression. Isoproterenol is typically initiated at 1.0 μg/min, targeting a 20% increase in heart rate or an absolute heart rate >90 bpm, titrated to hemodynamic response and suppression of recurrent ventricular arrhythmia.21,22
11. Screening of family members
No recommendations can be given to do ECG screening of the families of individuals with asymptomatic ER pattern or individuals with strong family history of ER or ER with VF. There are no established provocative tests to diagnose concealed ER in family members of ER syndrome patients, although preliminary observation suggest that the Valsalva maneuver may assist in identifying concealed ER cases.21,22
11.1. Treatment
Following are recommendations from latest Consensus document of HRS/ACC/ESC.22
11.2. Class I
-
1.
ICD implantation is recommended in patients with a diagnosis of ER syndrome who have survived a cardiac arrest.
11.3. Class II a
-
1.
Isoproterenol infusion can be useful in suppressing electrical/VT storms in patients with diagnosis of ER syndrome.
-
2.
Quinidine in addition to an ICD can be useful for secondary prevention and suppression of VT/VF in patients with a diagnosis of ER syndrome.
11.4. Class II b
-
1.
ICD implantation may be considered in symptomatic family members of ER syndrome, with history of syncope in the presence of ST segment elevation >1 mm in 2 or more inferior or lateral leads.
-
2.
ICD implantation may be considered in asymptomatic individuals who demonstrate a high-risk ER ECG pattern (high J-wave amplitude, horizontal/descending ST) in infero-posterior leads the presence of a strong family history of juvenile unexplained sudden death with or without a pathogenic mutation.
11.5. Class III
ICD implantation is not recommended in asymptomatic patients with an isolated ER pattern on ECG.
11.6. Conclusion
Clearly now ER pattern and ER syndrome are distinct entities, former being more common in young healthy individuals and athletes. Resuscitated VF with ER on ECG and symptomatic ER patients need to be treated with ICD. ER patterns with risk of degenerating into VF are being recognized; horizontal ST after notch being one, but there is no consensus yet. EP study for VT/VF induction is not useful and no provocative tests for induction of classical ER are available yet. Recommendations for sports participation and screening of family members are not yet defined but hopefully ongoing research shall show us light in near future.23
12. Brugada syndrome (BrS)
12.1. History
In 1992, Pedro and Josep Brugada published a landmark study describing eight sudden cardiac death patients in whom the ECG revealed “right bundle branch block” and ST segment elevation in precordial leads V1 to V3, without obvious structural heart diseases named this entity “Brugada syndrome.”24 In many cases of Brugada syndrome, the “right bundle branch block” appears without an S wave in the left precordial leads, suggesting that, in these cases, the right bundle branch block is apparent and that R represents an accentuation of the J wave. Second consensus conference report, published in 2005, focused on diagnosis, risk stratification schemes and approaches to therapy.25
12.2. Epidemiology
Limited data is available on the epidemiology of BrS. It is prevalent in Asian and Southeast Asian countries, especially Thailand, Philippines and Japan, reaching 0.5–1 per 1000. In some parts of Asia, BrS seems to be the most common cause of natural death in men younger than 50 years. BrS is known as Lai Tai (Thailand), Bangungut (Philippines), and Pokkuri (Japan). The reason for this higher prevalence in Asia is unknown. However, it has been speculated that it may be in part related to an Asian-specific sequence in the promoter region ofSCN5A.25–27
BrS is 8–10 times more prevalent in males than in females. The presence of a more prominent transient outward current (Ito) in males may be contributive. Higher testosterone levels also may have a significant role in the male predominance.25
12.3. Genetics of Brugada syndrome
BrS has an autosomal dominant mode of transmission with variable penetrance. Twelve responsible genes have been reported so far. In all 12 genotypes, either a decrease in the inward sodium or calcium current, or an increase in one of the outward potassium currents has been shown to be associated with the BrS phenotype. Genetic abnormalities are found in one third of genotyped BrS patients.SCN5A, the gene that encodes for the α subunit of the cardiac sodium channel, account for less than 30% of clinically diagnosed BrS patients.28
12.4. Clinical manifestations
BrS may be just an ECG detected accidental finding in an asymptomatic individual. Symptoms associated with BrS may include: Ventricular fibrillation (VF) or aborted sudden cardiac death (SCD), more often at night than during the day; presyncope or syncope; Nocturnal agonal respiration; episodic palpitation or atypical Chest pain.
These symptoms frequently occur during (i) febrile illness, (ii) rest or sleep or (iii) with vagotonic conditions, but rarely (iv) during exercise.
The syndrome typically manifests during adulthood, with a mean age of sudden death of 41 ± 15 years. BrS is associated with no clearly apparent structural heart diseases; however, several clinical studies have reported mild right and left ventricular structural abnormalities.25
12.5. Diagnosis
Following three types29 of ECG pattern are described (Fig. 6):
-
1.
Type 1 BrS is diagnosed in patients with ST segment elevation >2 mm in > one lead among the right precordial leads V1–V3, followed by negative T wave, positioned in the 2nd, 3rd or 4th intercostal space occurring either spontaneously or after provocative drug test with intravenous administration of Class I antiarrhythmic drugs.
-
2.
Type 2 BrS: characterized by saddleback type ST segment elevation of more than 2 mm a trough and a positive or biphasic T wave.
-
3.
Type 3 BrS: characterized by saddleback or coved type ST segment elevation <1 mm.
Fig. 6.
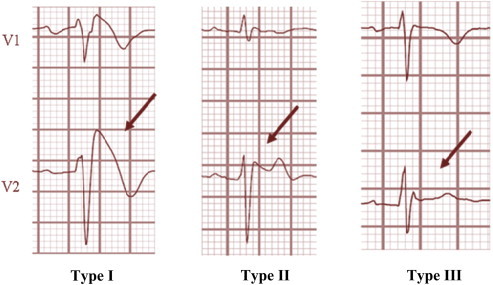
Type I II III Brugada syndrome.
Asymptomatic patients with type 1 changes do not require drug challenge. Other ECG abnormality consists of prolongation of PR, QRS and P wave duration and presence of S wave in lead I, II and III. There may be prolongation of QT interval in right precordial leads due to selective prolongation of action potential in right ventricular epicardium.22
Concealed ECG manifestations can be unmasked by febrile illness, sodium channel blockers and with vagotonic drugs.
Diagnosis of Brugada syndrome is made if Type 1 ST elevation is present with any one of following:
-
(i).
Documented ventricular fibrillation and/or polymorphic ventricular tachycardia.
-
(ii).
Inducible VT on EPS (Electrophysiology study).
-
(iii).
Syncope.
-
(iv).
Nocturnal agonal respiration.
-
(v).
Family history of sudden cardiac death in young individuals less than 45 years of age.
-
(vi).
ST elevation with T inversion in precordial leads in family members.
Type 2 and 3 are NOT diagnostic of BrS.22
Serial ECGs from the same patient may show all three patterns at different times, spontaneously or after administration of specific drugs.
Diagnosis of BrS should be considered if Type 2 or 3 changes convert to type I change on administration of sodium channel blocking drugs but conversion from type 3 to 2 on administration is NOT consistent with diagnosis of BrS.22
12.6. Provocative tests to unmask Brugada ECG pattern
Any of the following Sodium Channel Blocker drugs can induce Brugada changes and can be used in electrophysiology laboratory (EP Lab) (a). Procainamide 10 mg/kg IV over 10 min, (b). Flec-ainide 2 mg/kg IV over 10 min or 400 mg orally or (c) Ajmaline 1 mg/kg IV over 5 min or (d) Pilsicainide 1 mg/kg IV over 10 min31,32
12.7. Differential diagnosis (ECG mimics of BrS)
A number of diseases and conditions can lead to Brugada-like ECG abnormality(14) (i) Atypical RBBB, (ii)Left ventricular hypertrophy, (iii) Benign early repolarization, (iv)Acute Pericarditis, (v)Acute myocardial ischemia or infarction, (vi) acute stroke,(vii) Pulmonary embolism, (vii) Prinzmetal angina, (viii). Dissecting aortic aneurysm,(ix) various central and autonomic nervous system abnormalities, (x). Duchene muscular dystrophy, (xi) Thiamine deficiency, (xii). Hyperkalemia or hypocalcaemia, (xiii) Arrhythmogenic right ventricular cardiomyopathy (ARVC), (xiv) Pectus Excavatum, (xv) Hypothermia, and (xvi) Mechanical compression of the right ventricular outflow tract (RVOT) as occurs in mediastinal tumor or hemopericardium.
12.8. Asymptomatic type I Brugada
Many subjects displaying a type 1 ECG, spontaneous or drug-induced, are asymptomatic. In asymptomatic patients, the following findings are considered supportive for the diagnosis of BrS:
-
i.
Attenuation of ST segment elevation at peak of exercise stress test followed by its appearance during recovery phase.
-
ii.
Presence of first degree AV block and left axis deviation of the QRS.
-
iii.
Presence of atrial fibrillation.
-
iv.
Signal averaged ECG; Late potentials.
-
v.
Fragmented QRS.
-
vi.
ST-T alternans, spontaneous Ventricular Premature Beats of LBBB morphology during prolonged ECG recording.
-
vii.
Ventricular ERP <200 ms recorded during EPS and HV interval >60 m s.
-
viii.
Absence of structural heart disease including myocardial ischemia.
12.9. Prognosis and risk stratification
Following characteristics identify high risk patients:22
-
1.
Aborted Sudden Cardiac Death.
-
2.
Spontaneous and persistent Type I ST changes increase the risk of SCD by 8 fold.
-
3.
Inducible VT/VF on EPS. It also has 8 fold increase in risk of SCD.
-
4.
Syncope.
-
5.
Male gender: 5 fold increase in SCD.
12.10. Brugada-like ECG pattern inducing agents
Following drugs may cause Brugada-like ECG pattern.14
-
1.
Antiarrhythmic drugs: Flecainide, Propafenone, Ajmaline, Procainamide, Disopyramide.
-
2.
Calcium channel blockers like Verapamil, Nifedipine, Diltiazem.
-
3.
Beta blockers like Propranolol, Nadolol.
-
4.
Nitrates: isosobide dinitrate, Nitroglycerine.
-
5.
Potassium channel openers: Nicorandil.
-
6.
Tricyclic antidepressants: Amitryptaline, Nortriptyline, Desipramine, Clomipramide.
-
7.
Tetracyclic antidepressant: Maprotiline.
-
8.
Phenothiazines: Perphenazine, Cyamemazine.
-
9.
Selective serotonin reuptake inhibitors: Fluoxetine.
-
10.
Misc: Cocaine, Alcohol abuse.
12.11. Role of genetic testing
Diagnostic genetic testing can be considered21 for patients who clinically manifest with symptoms of BrS and for patients who are asymptomatic but are within a family with a known mutation. Testing should be performed first on the family member who is symptomatic and has clinical manifestations of BrS. Preferably; the youngest of most severely affected family members should be tested first. The three possible outcomes of genetic testing are: positive, negative, and variant of unknown clinical significance (VOUS). Identification of a mutation in the family can lead to genetic identification of at risk family members who are clinically asymptomatic and who may have normal ECG. Family members who test positive for the familial mutation should receive baseline electrocardiogram and annual ECG screening exams. Alternatively, a negative genetic test result for the familial mutation would obviate the need for repeated follow up examinations. Genetic testing can be used for prenatal diagnosis. All patients who undergo genetic testing should receive pre-test and post-test genetic counseling to understand the implications of testing.27–33
12.12. Catheter ablation of BrS
The underlying electrophysiological mechanism in patients with BrS is delayed depolarization over the anterior aspect of the RVOT epicardium. Catheter ablation over this abnormal area results in normalization of the Brugada ECG pattern and prevents VT/VF, both during electrophysiological studies as well as spontaneous recurrent VT/VF episodes.34,35 Epicardial radiofrequency ablation after pericardial access is in a state of evaluation and no clear-cut guidelines are available at this stage.
12.13. Treatment of Brugada syndrome
Following are guidelines/recommendations from latest consensus statement from HRS/ESC/ACC for treatment of BrS22:
12.14. Class I
-
1.The following lifestyle changes are recommended in all patients with diagnosis of BrS:
-
a)Avoiding of drugs that may induce or aggravate ST segment elevation in right precordial leads,
-
b)Avoiding of excessive alcohol intake,
-
c)Immediate treatment of fever with antipyretic drugs.
-
a)
-
2.ICD implantation is recommended only in patients with a diagnosis of BrS who:
-
a)Are survivors of a cardiac arrest, and/or
-
b)Have documented spontaneous sustained VT with or without syncope.
-
a)
12.15. Class II a
-
1.
ICD implantation can be useful in patients with a spontaneous diagnostic Type I ECG who have a history of syncope judged to be likely caused by ventricular arrhythmias.
-
2.
Quinidine can be useful in patients with a diagnosis of BrS and history of arrhythmic storms defined as more than two episodes of VT/VF in 24 h.
-
3.Quinidine can also be useful in patients with a diagnosis of BrS:
-
a)Who qualify for an ICD but present a contraindication to the ICD or refuse it, and/or
-
b)Have a history of documented supra-ventricular arrhythmia that requires treatment.
-
a)
-
4.
Isoproterenol infusion can be useful in suppressing VT storms in BrS patients.
12.16. Class II b
-
1.
ICD implantation may be considered in patients with a diagnosis of BrS who develop VF during programmed electrical stimulation (inducible patients).
-
2.
Quinidine may be considered in asymptomatic patients with a diagnosis of BrS with a spontaneous type 1 ECG.
-
3.
Catheter ablation may be considered in patients with a diagnosis of BrS and history of arrhythmic storms or repeated appropriate ICD shocks.
12.17. Class III
ICD implantation is not indicated in asymptomatic BrS patients with a drug-induced type I ECG and on the basis of a family history of SCD alone.
12.18. Conclusion
The cause of death in BrS is VF. The episodes of syncope (fainting) and sudden death or aborted sudden death are caused by fast polymorphic ventricular tachycardia or VF. These arrhythmias appear with no warning but fever probably is common occurrence. While there is no exact treatment modality that reliably and totally prevents ventricular fibrillation from occurring in this syndrome, treatment lies in termination of this lethal arrhythmia before it causes death and is done by implantation of an implantable cardioverter-defibrillator (ICD). Some recently performed studies had evaluated the role of quinidine, a Class IA antiarrhythmic drug, for decreasing VF episodes occurring in this syndrome. Quinidine was found to decrease number of VF episodes and correcting spontaneous ECG changes, possibly via inhibiting Itochannels. Some drugs have been reported to induce the type-1 ECG and/or (fatal) arrhythmias in Brugada syndrome patients. Patients with Brugada syndrome can prevent arrhythmias by avoiding these drugs, or use them only in controlled conditions. Those with risk factors for coronary artery disease may require a coronary angiogram before ICD implantation. J wave syndromes viz ER and BrS do have some similarities as shown in Table 1.
Table 1.
Common features of ER and BrS.
| Early repolarization | Brugada syndrome | |
|---|---|---|
|
35 | 35–40 |
|
75% | 75% |
|
Yes | Yes |
|
Yes | Yes |
|
Yes | Yes in type II and III |
|
Yes | Yes |
Conflicts of interest
All authors have none to declare.
References
- 1.Barnes A.R., Katz L.N., Levine S.A., Pardee H.E.B., White P.D., Wilson F.N. Report of the committee of American Heart Association on standardization of electrocardiographic nomenclature. Am Heart J. 1943;25:528–534. [Google Scholar]
- 2.Yan G.X., Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 3.Gussak I., Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M., Derval N., Sacher F. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 5.Shilpey R., Hallaran W. The four lead electrogram in 200 normal men and women. Am Heart J. 1936;11:325–345. [Google Scholar]
- 6.Tomaszewski W. Changement electrocardiographiques observes chez un home mort de froid. Arch Mal Coeur Vaiss. 1938;31:525–528. [Google Scholar]
- 7.Osborn J.J. Experimental hypothermia: respiratory and blood pH changes in relation to cardiac function. Am J Phys. 1953;175:389–398. doi: 10.1152/ajplegacy.1953.175.3.389. [DOI] [PubMed] [Google Scholar]
- 8.Wasserburger R.H., Alt W.J. The normal RS-T segment elevation variant. Am J Cardiol. 1961;8:184–192. doi: 10.1016/0002-9149(61)90204-1. [DOI] [PubMed] [Google Scholar]
- 9.Otto C.M., Tauxe R.V., Cobb L.A. Ventricular fibrillation causes sudden death in Southeast Asian immigrants. Ann Intern Med. 1984;101:45–47. doi: 10.7326/0003-4819-101-1-45. [DOI] [PubMed] [Google Scholar]
- 10.Gussak I., Antzelevitch C., Bjerregaard P., Towbin J.A., Chaitman B.R. The Brugada syndrome: clinical, electrophysiological and genetic aspects. J Am Coll Cardiol. 1999;33:5–15. doi: 10.1016/s0735-1097(98)00528-2. [DOI] [PubMed] [Google Scholar]
- 11.Kalla H., Yan G.X., Marinchak R. Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: a Brugada syndrome variant? J Cardiovasc Electrophysiol. 2000;11:95–98. doi: 10.1111/j.1540-8167.2000.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 12.Takagi M., Aihara N., Takaki H. Clinical characteristics of patients with spontaneous or inducible ventricular fibrillation without apparent heart disease presenting with J wave and ST segment elevation in inferior leads. J Cardiovasc Electrophysiol. 2000;11:844–848. doi: 10.1111/j.1540-8167.2000.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 13.Nam G.B., Kim Y.H., Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078–2079. doi: 10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antzelevitch C. Yan GX.J wave syndromes – contemporary review. Heart Rhythm. 2010;7:549–559. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junttila M.J., Sager S.J., Tikkanen J.T. Clinical significance of variants of J-points and J-waves: early repolarization patterns and risk. Eur Heart J. 2012 Nov;33:2639–2643. doi: 10.1093/eurheartj/ehs110. [DOI] [PubMed] [Google Scholar]
- 16.Haissaguerre M., Sacher F., Nogami A. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol. 2009;53:612–619. doi: 10.1016/j.jacc.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 17.Kambara H., Phillips J. Long-term evaluation of early repolarization syndrome (normal variant RS-T segment elevation) Am J Cardiol. 1976;38:157–161. doi: 10.1016/0002-9149(76)90142-9. [DOI] [PubMed] [Google Scholar]
- 18.Haissaguerre M., Chatel S., Sacher F. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros-Domingo A., Tan B.H., Crotti L. Gain of function mutation S422L in the KCNJ8- encoded cardiac KATP channel Kir6.1 as a pathogenic substrate for J wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burashnikov E., Pfeiffer R., Barajas—Martinez H. Mutations in cardiac L type channel associated with inherited J wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackerman M.J., Priori S.G., Willems S. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011 Aug;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 22.HRS/EHRA/APHRS Expert Consensus Statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. Oct 2013;Vol 10:No.10 doi: 10.1016/j.hrthm.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Tikkanen J.T., Anttonen O., Junttila M.J. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 24.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 25.Antzelevitch C., Brugada P., Borggrefe M. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 26.Munger R.G., Booton E.A. Bangungut in Manila: sudden and unexplained death in sleep of adult Filipinos. Int J Epidemiol. 1998;27:677–684. doi: 10.1093/ije/27.4.677. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control (CDC) Sudden, unexpected, nocturnal deaths among Southeast Asian refugees. MMWR Morb Mortal Wkly Rep. 1981;30:581–584. [PubMed] [Google Scholar]
- 28.Antzelevitch C., Yan G.X. Cellular and ionic mechanisms responsible for the Brugada syndrome. J Electrocardiol. 2000;33:33–39. doi: 10.1054/jelc.2000.20321. [DOI] [PubMed] [Google Scholar]
- 29.Bayés de Luna A., Brugada J., Baranchuk A. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012 Sep;45:433–442. doi: 10.1016/j.jelectrocard.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki T., Mitamura H., Miyoshi S., Soejima K., Aizawa Y., Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27:1061–1070. doi: 10.1016/0735-1097(95)00613-3. [DOI] [PubMed] [Google Scholar]
- 31.Brugada R., Brugada J., Antzelevitch C. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510–515. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 32.Morita H., Morita S.T., Nagase S. Ventricular arrhythmia induced by sodium channel blocker in patients with Brugada syndrome. J Am Coll Cardiol. 2003;42:1624–1631. doi: 10.1016/j.jacc.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Brugada J., Brugada R., Brugada P. Electrophysiologic testing predicts events in Brugada syndrome patients. Heart Rhythm. 2011;8:1595–1597. doi: 10.1016/j.hrthm.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Wilde A.A., Viskin S. EP testing does not predict cardiac events in Brugada Syndrome. Heart Rhythm. 2011;8:1598–1600. doi: 10.1016/j.hrthm.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa E., Takagi M., Tatsumi H., Yoshiyama M. Successful radiofrequency catheter ablation for electrical storm of ventricular fibrillation in a patient with Brugada syndrome. Circ J. 2008 Jun;72:1025–1029. doi: 10.1253/circj.72.1025. [DOI] [PubMed] [Google Scholar]


