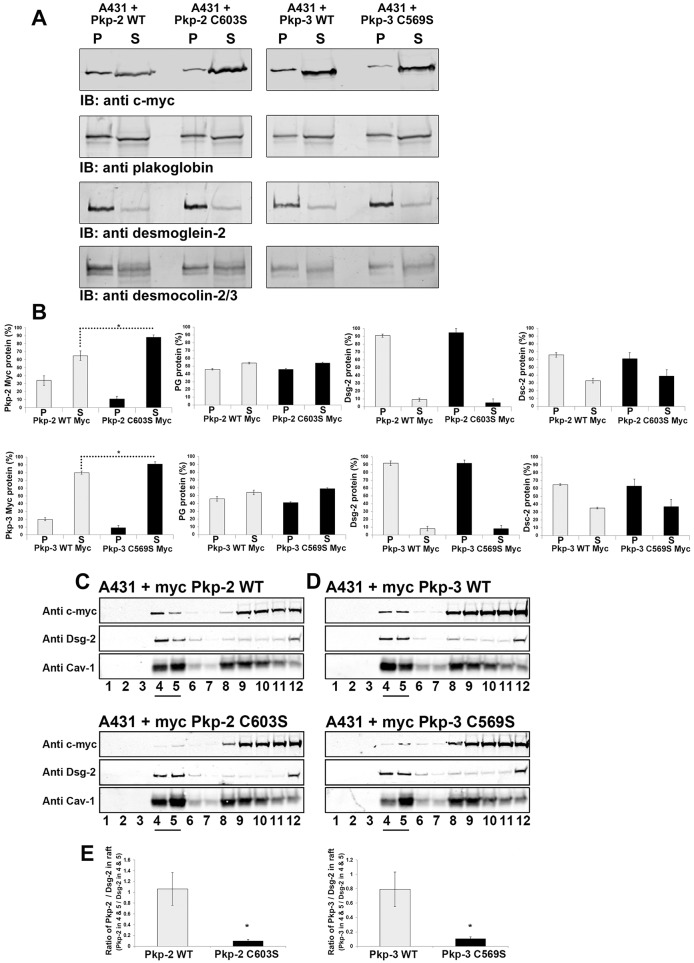
Fig. 6.
Plakophilin mutants display altered Triton X-100 solubility and lipid raft association compared with that of wild-type plakophilin. (A) Triton-X-100-insoluble (P) and soluble fractions (S) were prepared from A431 cells expressing wild-type plakophilin-2 (Pkp-2 WT), plakophilin-2 C603S (Pkp-2 C603S), wild-type plakophilin-3 (Pkp-3 WT) or plakophilin-3 C569S (Pkp-3 C569S). Immunoblot (IB) analysis was performed using antibodies against the Myc epitope tag, plakoglobin, desmoglein-2 and desmocollin-2/3. (B) Immunoblot analysis was performed, and the signal was quantified using a Li-Cor Odyssey Imaging system. Myc-tagged plakophilin-2 C603S and Myc-tagged plakophilin-3 C569S were found to be more soluble compared with Myc-tagged wild-type plakophilin-2 and plakophilin-3. The Triton X-100 solubility of endogenous plakoglobin (PG), desmoglein-2 (Dsg-2) and desmocollin-2/3 (Dsc-2) was not altered by the expression of mutant plakophilin-2 or plakophilin-3. (C,D) Cell lysates were prepared from A431 cells expressing Myc-tagged wild-type plakophilin-2 and Myc-tagged plakophilin-2 C603S (C) and Myc-tagged wild-type plakophilin-3 and Myc-tagged plakophilin-3 C569S (D). Lysates were subjected to sucrose-density centrifugation, 1-ml fractions were collected and immunoblot analysis was performed with the indicated antibodies. Fractions 4 and 5 (underlined) are enriched in the lipid raft component caveolin-1 (Cav-1), endogenous desmoglein-2 and wild-type plakophilin-2 and wild-type plakophilin-3. (E) The ratio of Myc signal to the desmoglein-2 signal present in fractions 4 and 5 is shown graphically. The ratios of plakophilin-2 C603S and plakophilin-3 C569S to the corresponding desmoglein-2 signal are reduced in fractions 4 and 5 compared with that of the wild-type plakophilins. For B,E, data show the mean±s.d. [n = 3 (B,E)]; *P<0.05.