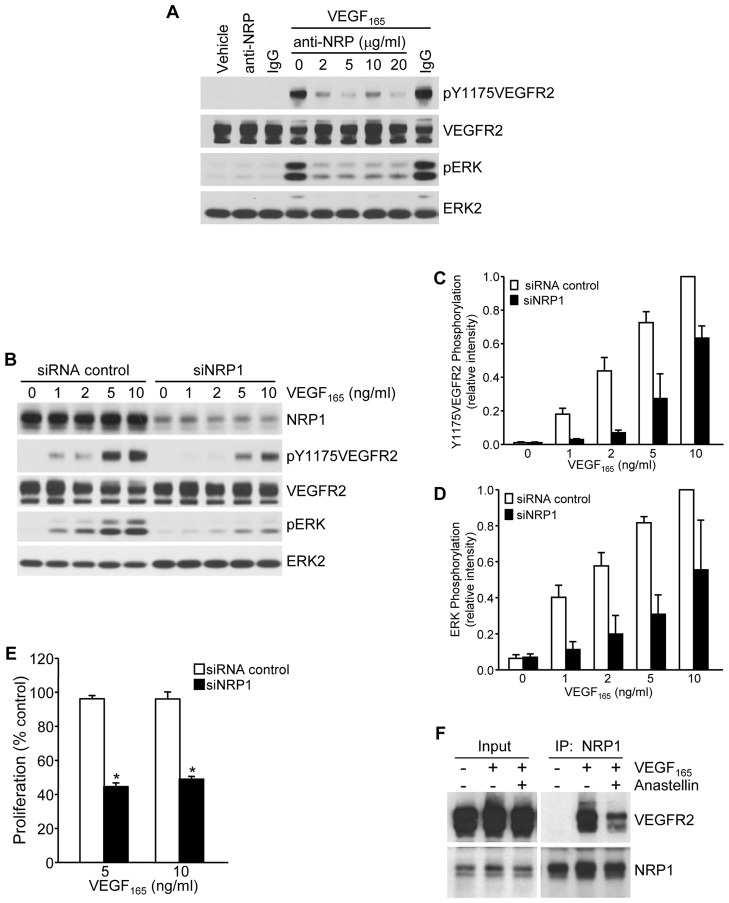
Fig. 2.
Anastellin disrupts VEGFR2–NRP1 complex assembly and signaling. (A) Serum-starved microvessel cell monolayers were pretreated for 2 hours with increasing concentrations of an NRP1-function-blocking antibody and then stimulated with 5 ng/ml VEGF165 for 6 minutes. Cell lysates were analyzed by western blotting for phosphorylation of VEGFR2 and ERK. (B) Microvessel cells treated with either siRNA against NRP1 or control siRNA were stimulated with increasing concentrations of VEGF165 for 6 minutes and analyzed for the phosphorylation of VEGFR2 and ERK by western blotting. Total VEGFR2 and ERK2 served as loading controls. (C,D) Blots from B were quantified using ImageJ software. Data show the mean±s.e.m. (three independent experiments). (E) Microvessel cells were grown for 3 days in basal medium containing the indicated amount of VEGF165. Cells were fixed and cell counts were obtained by Toluidine Blue staining. Knockdown of NRP1 significantly inhibited cell growth. Data show the mean±s.e.m. (three independent experiments); *P<0.001. (F) Microvessel cells were treated for 60 minutes with 20 µM anastellin prior to stimulation with 10 ng/ml VEGF165 for 15 minutes. Cell monolayers were washed, and NRP1 was immunoprecipitated (IP) from cell lysates. Immunoprecipitates were analyzed by western blotting for co-precipitation of VEGFR2. Input amounts of VEGFR2 and NRP1 present in lysates prior to immunoprecipitation are shown on the left.