Table 1.
Selected optimization studies
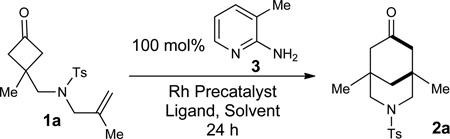 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Ligand/Additive | Solvent | Temp. (°C) |
Yield (%)* |
| 1 | 5 mol% [Rh(nbd)dppp]PF6 |
10 mol% BHT |
Xylenes | 130 | 0 |
| 2 | 10 mol% [Rh(COD)Cl]2 |
Toluene | 130 | <5 | |
| 3 | 10 mol% [Rh(COD)Cl]2 |
CIPh | 150 | 23(34) | |
| 4 | 10 mol% [Rh(COD)Cl]2 |
Xylenes | 150 | 27(30) | |
| 5 | 10 mol% [Rh(COD)Cl]2 |
1,4-Dioxane | 150 | 43(51) | |
| 6 | 10 mol% [Rh(COD)Cl]2 |
20 mol% COD |
1,4-Dioxane | 150 | 47(57) |
| 7 | 10 mol% [Rh(COD)Cl]2 |
20 mol% P(C6F5)3 |
1,4-Dioxane | 150 | 58(65) |
| 8 | 10 mol% [Rh(COD)Cl]2 |
10 mol% P(3,5-C6H3(CF3)2)3 |
1,4-Dioxane | 150 | 67(74) |
| 9 | 10 mol% [Rh(COD)Cl]2 |
22 mol% P(3,5-C6H3(CF3)2)3 |
1,4-Dioxane | 150 | 82 |
| 10 | 10 mol% [Rh(COD)Cl]2† |
22 mol% P(3,5-C6H3(CF3)2)3 |
1,4-Dioxane | 150 | 76(88) |
| 11 |
5 mol% [Rh(C2H4)2Cl]2 |
24 mol% P(3,5-C6H3(CF3)2)3§ |
1,4-Dioxane | 150 | 87 |
| 12 | 5 mol% [Rh(C2H4)2Cl]2‡ |
24 mol% P(3,5-C6H3(CF3)2)3§ |
1,4-Dioxane | 150 | 0‖ |
Isolated yield; numbers in parentheses are brsm yield.
20 mol% 3 was used with 71 h reaction time.
no 3 was added.
The reaction time was 48 h.
Decarbonylation products were observed as an inseperable mixture.
