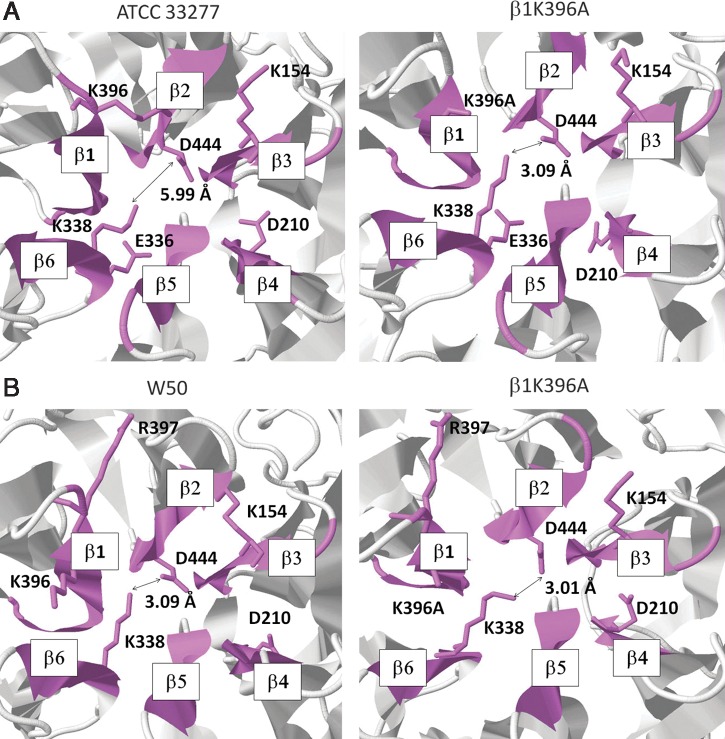
FIG. 3.
β1K396 residue influences the P. gingivalis sialidase β-propeller domain β2D444–β6K338 distance measurement. Sialidase β-propeller domains of (A) ATCC 33277 and (B) W50. (Left panel) original ATCC 33277 and W50 (β1K396) and (Right panel) altered ATCC 33277 and W50 (β1K396A) sialidase β-propeller domains. Potential salt bridge-forming residues found in the sialidase β-propeller domain are in wireframe orientation. Six β-sheets found in the sialidase β-propeller domain are labeled as β1–6 following a clockwise orientation. Salt bridge distance measurement is indicated in angstrom (Å).