Fig. 7.
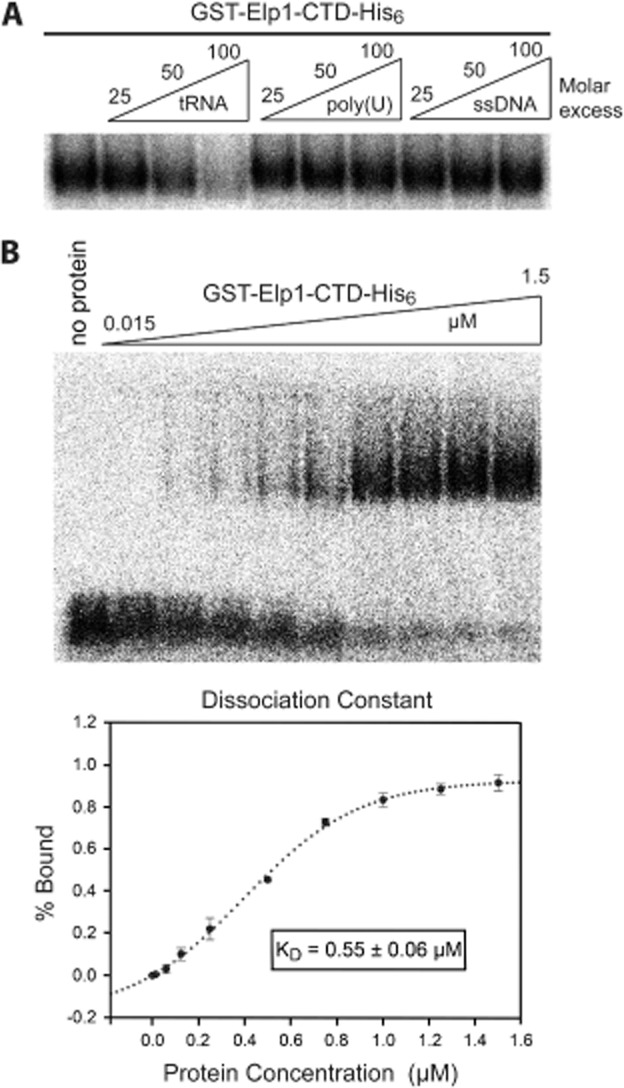
Binding of the Elp1 C-terminal domain to tRNA occurs with a dissociation constant in the low micromolar range and is not competed by poly(U) or ssDNA.
A. Electrophoretic mobility shift assay (EMSA) using ∼ 1 nM 32P-labelled yeast tRNA, 0.4 μM recombinant Elp1 C-terminal domain and the indicated molar excess of unlabelled yeast tRNA, poly(U) or ssDNA.
B. EMSA using ∼ 0.1 nM 32P-labelled yeast tRNA and the indicated concentrations of recombinant Elp1 C-terminal domain. Bound and free tRNA bands were quantified from three replicate experiments and used to estimate a dissociation constant (KD) of 0.55 μM for the interaction by non-linear curve fitting (f = a*xb/[cb + xb]). The Hill coefficient (b) for the fitted curve was 1.76 ± 0.19. All binding reactions were separated on a 1.5% native agarose gel and analysed by autoradiography.
