Abstract
Aim
The aim of this report is to provide initial evidence that add-on treatment with perampanel might be highly effective in progressive myoclonic epilepsy such as Lafora disease.
Case report
We report on a 21-year-old woman suffering from persistent myoclonus and generalized tonic–clonic seizures for more than seven years. Additionally, ataxia, a disturbance in speech and gait, as well as a cognitive decline were rapidly progressing. Subsequently, the diagnosis of Lafora disease was confirmed by the identification of a novel homozygous missense mutation in exon 3 of the EPM2A gene (c.538C>G; p.L180V).
Adjunctive therapy with perampanel was started in this patient with advanced Lafora disease and was titrated up to 8 mg/day. A sustained and reproducible remission of myoclonus and GTCS could be achieved for a follow-up of three months. After dosage reduction to 6 mg/day, seizures recurred; however, on increasing the daily dose to 10 mg, seizures stopped for another three months. The patient also regained her ability to walk with help and the aid of a walker.
Conclusions
Perampanel is a selective, noncompetitive antagonist of AMPA-type glutamate receptors and recently licensed as adjunctive therapy for the treatment of refractory focal onset seizures. There is evidence for its effectiveness in generalized epilepsies, and phase III studies for this indication are on the way. Our case illustrates the possibility that perampanel might be a valuable option for treatment in PME. Considering its impressive efficacy in this case, we suggest a prospective, multicenter study evaluating perampanel in PME.
Keywords: Perampanel, Epilepsy, Progressive myoclonic epilepsy, Lafora, Myoclonus, EPM2A
1. Introduction
The treatment of patients with Lafora disease (LD), a form of progressive myoclonus epilepsies (PME), proves to be very difficult, and previously healthy children or teenagers are afflicted with ever-worsening and soon-intractable myoclonus and epilepsy, which are usually associated with dementia and early death [1,2].
Lafora disease is an autosomal recessive, most commonly teenage-onset neurodegenerative disorder caused by mutations in either the EPM2A or NHLRC1 gene, encoding the interacting proteins laforin and malin, respectively. Patients with LD usually present between 8 and 18 years of age with an insidious near-simultaneous or closely consecutive appearance of headaches, difficulties in school work, myoclonus, generalized tonic–clonic seizures (GTCS), and visual hallucinations of both epileptic and psychotic origin. For many years, patients struggle to maintain normal contact and communication, interrupted by extremely frequent myoclonic absence seizures. Gradually, dementia sets in, and patients are in an almost continuous myoclonus with absences, frequent GTCS, and profound dementia or vegetative state within a decade of onset [1–3].
We report a case in which perampanel was started in an adult patient with advanced LD leading to sustained remission in myoclonus and generalized tonic–clonic seizures.
2. Case report
A 14-year-old, normally developed girl of Turkish descent initially presented to our clinic with myoclonus and GTCS. During video-EEG monitoring, interictal generalized spikes and polyspikes as well as three seizures with generalized onset were recorded. There was no evidence of focal EEG seizure onset. Over the next 7 years, no seizure remission had been achieved on various combinations of antiepileptic drugs or treatment with vagus nerve stimulation. Follow-up at the age of 18 years showed mild generalized and hippocampal atrophy on MRI (Fig. 1A–D). Neuropsychological evaluation showed a decline in language skills, especially in German, her second language. The patient spoke slowly and only few words with perseverations. Her attendance at a special school had decreased because of the seizures. No standardized neuropsychological testing was possible. The patient could name colors correctly and was interested in details of shown pictures. We observed a decline in language skills and attention, but motivational skills remained as a cognitive resource. Since then, disturbance in gait (ataxia) and cognitive decline were rapidly progressing, and eventually, the patient lost her ability to speak.
Fig. 1.
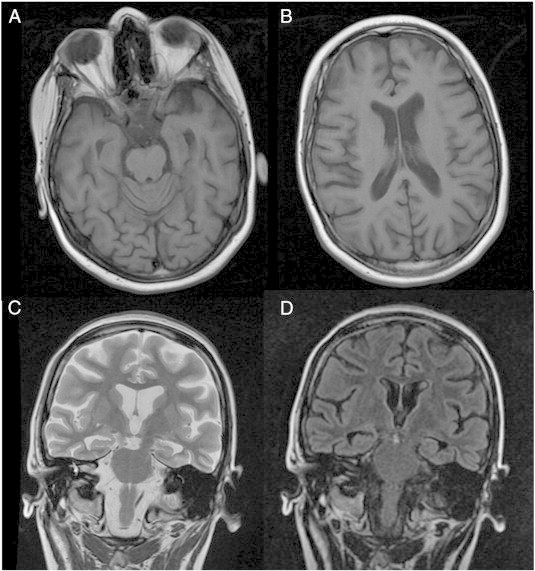
T1, T2, and FLAIR MRI with mild generalized atrophy (A, B) as well as bilateral hippocampal atrophy (C, D).
The suspected diagnosis of Lafora disease was confirmed by the identification of a novel homozygous missense mutation in exon 3 of the EPM2A gene (c.538C>G; p.L180V) by Sanger sequencing, which was heterozygous in her unaffected parents.
In April 2012, the 21-year-old patient was admitted with a generalized convulsive status epilepticus (SE) that proved super-refractory [4]. Therapy with thiopental anesthesia, steroids, and magnesium did not control the SE, and a termination of all therapy was considered. Finally, initiation and maintenance of a ketogenic diet terminated the SE, as reported earlier [5], and the patient was discharged. She was bedbound and continued to suffer from daily myoclonus and frequent GTCS every 3–4 days despite antiepileptic polytherapy with high doses of valproate, levetiracetam, clonazepam, piracetam, and zonisamide and ketogenic diet.
Subsequently, the patient was admitted in September 2012 because of seizure exacerbation caused by infection and fluoroquinolone antibiotic therapy. The EEG showed generalized epileptiform discharges and frequent myoclonus (Fig. 2). The patient was started on an adjunctive therapy with the newly licensed perampanel, which was quickly titrated up to 8 mg/day within one week. No side effects were observed. The other anticonvulsant agents, such as valproate (1350 mg/day), levetiracetam (4000 mg/day), zonisamide (600 mg/day), clonazepam (12 mg/day), piracetam (12,000 mg/day), and the ketogenic diet (4:1 ratio) were not changed at this point in time. The GTCS stopped, and the patient was discharged. On follow-up four months later, the parents reported sustained seizure remission without myoclonus and GTCS for more than 3 months on an unchanged therapeutic regimen (see Table 1). The EEG showed less epileptiform discharges (Fig. 3). The patient was able to walk with help and the aid of a walker and could climb a few stairs during physiotherapy. A few weeks later, the parents reported gait problems, and therefore, perampanel was decreased from 8 mg/day to 6 mg/day. Generalized tonic–clonic seizures recurred, but control was again achieved after increasing perampanel to 10 mg/day with a follow-up of another three months.
Fig. 2.
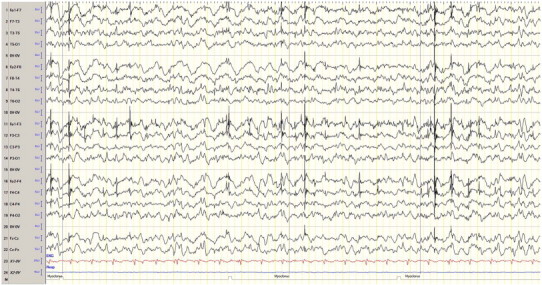
EEG with generalized epileptiform discharges and associated myoclonus before initiation of perampanel.
Table 1.
Antiepileptic therapy and serum concentrations during seizure remission.
| Anticonvulsant | Daily dose | Serum concentration | Therapeutic level |
|---|---|---|---|
| Clonazepam | 12 mg | n.a. | n.a. |
| Levetiracetam | 4000 mg | 22.3 mg/l | 3–34 mg/l |
| Perampanel | 8 mg | 442 μg/l | 100–800 μg/l |
| Piracetam | 12,000 mg | 52 μg/ml | n.a. |
| Valproate | 1350 mg | 69.8 μg/ml | 40–100 μg/ml |
| Zonisamide | 600 mg | 21.6 μg/ml | 10–40 μg/ml |
| Ketogenic diet | 4:1 ratio |
Fig. 3.
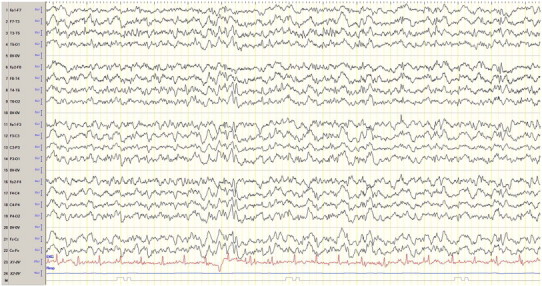
EEG with bifrontal polyspikes and generalized slowing on follow-up.
3. Discussion
Perampanel was used off-label in this patient with advanced LD and resulted in a sustained and reproducible remission of myoclonus and GTCS. Transient dose reduction of perampanel caused the reoccurrence of seizures, proving the causal relationship of therapy with perampanel and seizure freedom in this progressive disorder.
Perampanel is a selective, noncompetitive antagonist of AMPA-type glutamate receptors and was recently licensed as adjunctive therapy for the treatment of refractory focal onset seizures [6–8]. Perampanel exhibited broad-spectrum efficacy in various animal seizure models, and there is evidence for its effectiveness in generalized epilepsies [9]. A phase 3 trial to determine the efficacy and safety of adjunctive perampanel in primary GTCS is currently recruiting patients (ClinicalTrials.gov identifier: NCT01393743). The reason for the striking efficacy in our patient with LD remains elusive and requires further study.
New treatment options are desired in PME such as LD and Unverricht–Lundborg disease [1,2] to improve daily functioning and seizure control. Our case illustrates that perampanel might be a valuable option for treatment in PME. Considering its impressive efficacy in this case, we suggest a prospective, multicenter study evaluating perampanel in PME.
Conflict of interest statement
K. Schorlemmer, Dr. S. Bauer, M. Belke, A. Hermsen, Dr. K.M. Klein, and Prof. Dr. W.S. Kunz report no disclosures. Dr. P.S. Reif has received travel support from UCB Pharma. Prof. Dr. W.H. Oertel has received honoraria and/or research grants from Boehringer Ingelheim, Desitin, GlaxoSmithKline, Merck, Mundipharma, Novartis, Orion, Sharp & Dohme, Schwarz Pharma Neuroscience/UCB Pharma, Synosia, and Teva. Prof. Dr. S. Knake has received honoraria from Desitin and UCB Pharma. Prof. Dr. F. Rosenow has received honoraria and/or support for teaching courses from Cerbomed, Cyberonics, Desitin, Eisai, GlaxoSmithKline, Medtronic, Nihon-Kohden, Pfizer, and UCB Pharma. Dr. A. Strzelczyk has received travel support and honoraria from Desitin, Eisai, and UCB Pharma.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Girard J.M., Turnbull J., Ramachandran N., Minassian B.A. Progressive myoclonus epilepsy. Handb Clin Neurol. 2013;113:1731–1736. doi: 10.1016/B978-0-444-59565-2.00043-5. [DOI] [PubMed] [Google Scholar]
- 2.Ramachandran N., Girard J.M., Turnbull J., Minassian B.A. The autosomal recessively inherited progressive myoclonus epilepsies and their genes. Epilepsia. 2009;50(Suppl. 5):29–36. doi: 10.1111/j.1528-1167.2009.02117.x. [DOI] [PubMed] [Google Scholar]
- 3.Lesca G., Boutry-Kryza N., de Toffol B., Milh M., Steschenko D., Lemesle-Martin M. Novel mutations in EPM2A and NHLRC1 widen the spectrum of Lafora disease. Epilepsia. 2010;51:1691–1698. doi: 10.1111/j.1528-1167.2010.02692.x. [DOI] [PubMed] [Google Scholar]
- 4.Shorvon S., Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134:2802–2818. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 5.Strzelczyk A., Reif P.S., Bauer S., Belke M., Oertel W.H., Knake S. Intravenous initiation and maintenance of ketogenic diet: proof of concept in super-refractory status epilepticus. Seizure. 2013;22:581–583. doi: 10.1016/j.seizure.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 6.French J.A., Krauss G.L., Biton V., Squillacote D., Yang H., Laurenza A. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79:589–596. doi: 10.1212/WNL.0b013e3182635735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krauss G.L., Serratosa J.M., Villanueva V., Endziniene M., Hong Z., French J. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78:1408–1415. doi: 10.1212/WNL.0b013e318254473a. [DOI] [PubMed] [Google Scholar]
- 8.Steinhoff B.J., Ben-Menachem E., Ryvlin P., Shorvon S., Kramer L., Satlin A. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies. Epilepsia. 2013;54:1481–1489. doi: 10.1111/epi.12212. [DOI] [PubMed] [Google Scholar]
- 9.Rogawski M.A., Hanada T. Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol Scand Suppl. 2013;197:19–24. doi: 10.1111/ane.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]


